Most Common Substances in Chemicals for Different Industries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Summary Tables of Calculated and Experimental Parameters Of
Summary Tables of Calculated and Experimental Parameters of Diatomic, Triatomic, Organic, Silicon, Boron, Aluminum and Organometallic Molecules, Exemplary Results on Condensed Matter Physics, One-Through Twenty-Electron Atoms, Excited States of Helium, g Factor, and Fundamental Particle Masses The closed-form derivations from Maxwell's equations given in The Grand Unified Theory of Classical Physics posted at http://www.blacklightpower.com/theory/bookdownload.shtml contain fundamental constants only. The nature of the chemical bond is given in Chapters 12 through 15. The atoms are solved exactly in Chapters 1, 7, and 10. The excited states of helium are solved exactly in Chapter 9. The electron g factor and relations between fundamental particles are given in Chapter 1 and Chapters 36, 37 and 38. Tables summarizing the results of the calculated experimental parameters of 800 exemplary solved molecules follow. The closed-form derivations of these molecules can be found in The Grand Theory of Classical Physics posted at http://www.blacklightpower.com/theory/bookdownload.shtml Chapters 15–17, as well as Silicon in Chapter 20, Boron in Chapter 22, and Aluminum and Organometallics in Chapter 23. Condensed matter physics based on first principles with analytical solutions of (i) of the geometrical parameters and energies of the hydrogen bond of H2O in the ice and steam phases, and of H2O and NH3; (ii) analytical solutions of the geometrical parameters and interplane van der Waals cohesive energy of graphite; (iii) analytical solutions of the geometrical parameters and interatomic van der Waals cohesive energy of liquid helium and solid neon, argon, krypton, and xenon are given in Chapter 16. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

A Chemical Kinetics Network for Lightning and Life in Planetary Atmospheres P
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by St Andrews Research Repository The Astrophysical Journal Supplement Series, 224:9 (33pp), 2016 May doi:10.3847/0067-0049/224/1/9 © 2016. The American Astronomical Society. All rights reserved. A CHEMICAL KINETICS NETWORK FOR LIGHTNING AND LIFE IN PLANETARY ATMOSPHERES P. B. Rimmer and Ch Helling School of Physics and Astronomy, University of St Andrews, St Andrews, KY16 9SS, UK; [email protected] Received 2015 June 3; accepted 2015 October 22; published 2016 May 23 ABSTRACT There are many open questions about prebiotic chemistry in both planetary and exoplanetary environments. The increasing number of known exoplanets and other ultra-cool, substellar objects has propelled the desire to detect life andprebiotic chemistry outside the solar system. We present an ion–neutral chemical network constructed from scratch, STAND2015, that treats hydrogen, nitrogen, carbon, and oxygen chemistry accurately within a temperature range between 100 and 30,000 K. Formation pathways for glycine and other organic molecules are included. The network is complete up to H6C2N2O3. STAND2015 is successfully tested against atmospheric chemistry models for HD 209458b, Jupiter, and the present-day Earth using a simple one- dimensionalphotochemistry/diffusion code. Our results for the early Earth agree with those of Kasting for CO2,H2, CO, and O2, but do not agree for water and atomic oxygen. We use the network to simulate an experiment where varied chemical initial conditions are irradiated by UV light. The result from our simulation is that more glycine is produced when more ammonia and methane is present. -

Directed Gas Phase Formation of Silicon Dioxide and Implications for the Formation of Interstellar Silicates
ARTICLE DOI: 10.1038/s41467-018-03172-5 OPEN Directed gas phase formation of silicon dioxide and implications for the formation of interstellar silicates Tao Yang 1,2, Aaron M. Thomas1, Beni B. Dangi1,3, Ralf I. Kaiser 1, Alexander M. Mebel 4 & Tom J. Millar 5 1234567890():,; Interstellar silicates play a key role in star formation and in the origin of solar systems, but their synthetic routes have remained largely elusive so far. Here we demonstrate in a combined crossed molecular beam and computational study that silicon dioxide (SiO2) along with silicon monoxide (SiO) can be synthesized via the reaction of the silylidyne radical (SiH) with molecular oxygen (O2) under single collision conditions. This mechanism may provide a low-temperature path—in addition to high-temperature routes to silicon oxides in circum- stellar envelopes—possibly enabling the formation and growth of silicates in the interstellar medium necessary to offset the fast silicate destruction. 1 Department of Chemistry, University of Hawai’iatMānoa, Honolulu, HI 96822, USA. 2 State Key Laboratory of Precision Spectroscopy, East China Normal University, Shanghai, 200062, China. 3 Department of Chemistry, Florida Agricultural and Mechanical University, Tallahassee, FL 32307, USA. 4 Department of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, USA. 5 Astrophysics Research Centre, School of Mathematics and Physics, Queen’s University Belfast, Belfast, BT7 1NN, UK. Correspondence and requests for materials should be addressed to R.I.K. (email: [email protected]) or to A.M.M. (email: mebela@fiu.edu) or to T.J.M. (email: [email protected]) NATURE COMMUNICATIONS | (2018) 9:774 | DOI: 10.1038/s41467-018-03172-5 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-03172-5 — 28 + 28 + he origin of interstellar silicate grains nanoparticles ( SiO2 ), and 44 ( SiO ). -

Gas Phase Formation of C-Sic3 Molecules in the Circumstellar Envelope of Carbon Stars
Gas phase formation of c-SiC3 molecules in the circumstellar envelope of carbon stars Tao Yanga,b,1,2, Luke Bertelsc,1, Beni B. Dangib,3, Xiaohu Lid,e, Martin Head-Gordonc,2, and Ralf I. Kaiserb,2 aState Key Laboratory of Precision Spectroscopy, East China Normal University, Shanghai 200062, P. R. China; bDepartment of Chemistry, University of Hawai‘iatManoa, Honolulu, HI 96822; cDepartment of Chemistry, University of California, Berkeley, CA 94720; dXinjiang Astronomical Observatory, Chinese Academy of Sciences, Urumqi, Xinjiang 830011, P. R. China; and eNational Astronomical Observatories, Chinese Academy of Sciences, Beijing 100012, P. R. China Contributed by Martin Head-Gordon, May 17, 2019 (sent for review July 20, 2018; reviewed by Piergiorgio Casavecchia and David Clary) Complex organosilicon molecules are ubiquitous in the circumstel- Modern astrochemical models propose that the very first lar envelope of the asymptotic giant branch (AGB) star IRC+10216, silicon–carbon bonds are formed in the inner envelope of the but their formation mechanisms have remained largely elusive until carbon star, which is undergoing mass loss at the rates of several − now. These processes are of fundamental importance in initiating a 10 5 solar masses per year (22, 23). Pulsations from the central chain of chemical reactions leading eventually to the formation of star may initiate shocks, which (photo)fragment the circumstellar — organosilicon molecules among them key precursors to silicon car- materials (24, 25). These nonequilibrium conditions cause tem- — bide grains in the circumstellar shell contributing critically to the peratures of 3,500 K or above (19) and lead to highly reactive galactic carbon and silicon budgets with up to 80% of the ejected metastable fragments such as electronically excited silicon atoms, materials infused into the interstellar medium. -
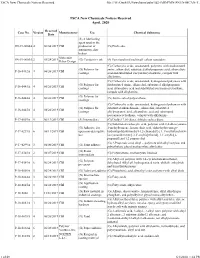
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

Reaction Pathways Linking Chemisorption to Desorption of Methylchlorosilanes on Copper(001)
REACTION PATHWAYS LINKING CHEMISORPTION TO DESORPTION OF METHYLCHLOROSILANES ON COPPER(001) BY JAMES LALLO A dissertation submitted to the Graduate School|New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry and Chemical Biology Written under the direction of Professor B.J. Hinch and approved by New Brunswick, New Jersey October, 2012 ABSTRACT OF THE DISSERTATION Reaction pathways linking chemisorption to desorption of methylchlorosilanes on copper(001) by James Lallo Dissertation Director: Professor B.J. Hinch The interactions of silane molecules with metal surfaces are pivotal in many com- mercial processes. Of particular interest is the commercial \Direct Process," in which methyl chloride (CH3Cl) is exposed to silicon, in the presence of a copper catalyst and other promoters, producing dimethyldichlorosilane as the predominant product. Previous UHV studies have investigated the interaction of \pre-dissociated" methyl (CH3) and chlorine (Cl) with copper and copper-silicide surfaces. This thesis investi- gates the reaction mechanisms of \pre-associated" methylchlorosilanes ((CH3)xClySiHz, x+y+z=4) adsorbed on a copper(001) surface. The goal was to develop an understand- ing of the intermediates and transfer processes involved for dissociative adsorption and subsequent desorption of these molecules. Only molecules containing at least one Si- H bond were observed to undergo chemisorption. It was found that dimethylsilane (CH3)2SiH2 and methylsilane (CH3)SiH3 exhibit ligand transfer on the copper sur- face, leading to the desorption of trimethylsilane (CH3)3SiH, in both cases. The sug- gested intermediates present after adsorption of methylsilane were methylsilyl CH3SiH2, methysilylene CH3SiH and methylsilylidyne CH3Si. -

Sih; X2Π) with Dimethylacetylene (CH3CCCH3; X1a1g
Article pubs.acs.org/JPCA Formation of the 2,3-Dimethyl-1-silacycloprop-2-enylidene Molecule via the Crossed Beam Reaction of the Silylidyne Radical (SiH; X2Π) 1 with Dimethylacetylene (CH3CCCH3;XA1g) Tao Yang, Aaron M. Thomas, Beni B. Dangi, and Ralf I. Kaiser* Department of Chemistry, University of Hawai’i at Manoa, Honolulu, Hawaii 96822, United States Mei-Hung Wu, Bing-Jian Sun, and Agnes H. H. Chang* Department of Chemistry, National Dong Hwa University, Shoufeng, Hualien 974, Taiwan *S Supporting Information ABSTRACT: We carried out crossed molecular beam experiments and electronic structure calculations to unravel the chemical dynamics of the 2Π reaction of the silylidyne(-d1) radical (SiH/SiD; X ) with dimethyla- 1 cetylene (CH3CCCH3;XA1g). The chemical dynamics were indirect and initiated by the barrierless addition of the silylidyne radical to both carbon atoms of dimethylacetylene forming a cyclic collision complex 2,3-dimethyl-1-silacyclopropenyl. This complex underwent unimolecular decomposition by atomic hydrogen loss from the silicon atom via a loose exit transition state to form the novel 2,3-dimethyl-1-silacycloprop-2- enylidene isomer in an overall exoergic reaction (experimentally: −29 ± 21 kJ mol−1; computationally: −10 ± 8 kJ mol−1). An evaluation of the scattering dynamics of silylidyne with alkynes indicates that in each system, the silylidyne radical adds barrierlessly to one or to both carbon atoms of the acetylene moiety, yielding an acyclic or a cyclic collision complex, which can also be accessed via cyclization of the acyclic structures. The cyclic intermediate portrays the central decomposing complex, which fragments via hydrogen loss almost perpendicularly to the rotational plane of the decomposing complex exclusively from the silylidyne moiety via a loose exit transition state in overall weakly exoergic reaction leading to ((di)methyl-substituted) 1-silacycloprop-2-enylidenes (−1to−13 kJ mol−1 computationally; −12 ± 11 to −29 ± 21 kJ mol−1 experimentally). -

SAFETY DATA SHEET Sodium Persulfate
SAFETY DATA SHEET Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Format: NA Version 1.06 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name Sodium Persulfate CAS-No 7775-27-1 Synonyms Sodium Peroxydisulfate; Disodium Peroxydisulfate; Peroxydisulfuric acid, disodium salt; Peroxydisulfuric acid, sodium salt. Recommended use of the chemical and restrictions on use Recommended Use: Polymerization initiator; Etchant and cleaner for printed circuit boards; Hair bleaching formulations; Secondary oil recovery; Oxidizing agent for a variety of organic reactions. Restrictions on Use No uses to be advised against were identified. Manufacturer/Supplier PeroxyChem LLC 2005 Market Street Suite 3200 Philadelphia, PA 19103 Phone: +1 267/ 422-2400 (General Information) E-Mail: [email protected] Emergency telephone numbers For leak, fire, spill or accident emergencies, call: 1 800 / 424 9300 (CHEMTREC - U.S.A.) 1 703 / 527 3887 (CHEMTREC - Collect - All Other Countries) +1 303/ 389-1409 (Medical - U.S. - Call Collect) Page 1 / 10 Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Version 1.06 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2B Respiratory sensitization Category 1 Skin sensitization Category 1 Specific target organ toxicity (single exposure) Category 3 Oxidizing Solids -
![Arxiv:1510.07052V1 [Astro-Ph.EP] 23 Oct 2015 99 Lae Ta.20,Adrfrne Therein)](https://docslib.b-cdn.net/cover/7858/arxiv-1510-07052v1-astro-ph-ep-23-oct-2015-99-lae-ta-20-adrfrne-therein-2397858.webp)
Arxiv:1510.07052V1 [Astro-Ph.EP] 23 Oct 2015 99 Lae Ta.20,Adrfrne Therein)
A Chemical Kinetics Network for Lightning and Life in Planetary Atmospheres P. B. Rimmer1 and Ch Helling School of Physics and Astronomy, University of St Andrews, St Andrews, KY16 9SS, United Kingdom ABSTRACT There are many open questions about prebiotic chemistry in both planetary and exoplane- tary environments. The increasing number of known exoplanets and other ultra-cool, substellar objects has propelled the desire to detect life and prebiotic chemistry outside the solar system. We present an ion-neutral chemical network constructed from scratch, Stand2015, that treats hydrogen, nitrogen, carbon and oxygen chemistry accurately within a temperature range between 100 K and 30000 K. Formation pathways for glycine and other organic molecules are included. The network is complete up to H6C2N2O3. Stand2015 is successfully tested against atmo- spheric chemistry models for HD209458b, Jupiter and the present-day Earth using a simple 1D photochemistry/diffusion code. Our results for the early Earth agree with those of Kasting (1993) for CO2, H2, CO and O2, but do not agree for water and atomic oxygen. We use the network to simulate an experiment where varied chemical initial conditions are irradiated by UV light. The result from our simulation is that more glycine is produced when more ammonia and methane is present. Very little glycine is produced in the absence of any molecular nitrogen and oxygen. This suggests that production of glycine is inhibited if a gas is too strongly reducing. Possible applications and limitations of the chemical kinetics network are also discussed. Subject headings: astrobiology — atmospheric effects — molecular processes — planetary systems 1. Introduction The input energy source and the initial chem- istry have been varied across these different ex- The potential connection between a focused periments. -

19650003847.Pdf
NATIONAL BUREAU OF STANDARDS REPO RT 8595 PRELIMINARY REPORT ON A SURVEY OF THERMODYNAMIC PROPERTIES OF THE COMPOUNDS OF THE ELEMENTS CHNOPS i N65 2 CACC£S$IO NUMB£:R) CTHRU) ~ ___f£ / I- (PAGES) (CdOEI ::; ~ CIU - d -P?02c2/ :33 (NASA CR OR TMX OR AD NUMBER) (CATEQORY) Progress Report for the Period 1 August to 31 October 1964 to National Aeronautics and Space Administration GPO PRICE $ _____ OTS PRICE(S) $ 1 November 1964 Hard copy (He) of· cJV r Microfiche (MF) __....;... : .:::.j~I?J~_ <@> U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARD S The ational Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. It responsibilities include development and main tena nce of the national stand· ards of measurement, and the provisions of means for making measurements consi tent with those standard ; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be nece sary, particu larl: for ~overn ment agencies; cooperation in the establi hment of standard practices for incorpora tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of device to serve special needs of the Government: assi tance to indus! r). business. and consumers in the development and acceptance of commercial tandard and simplified trade practice recommendations; administration of programs in cooperation with United tates bu iness groups and standards organizations for the development of international standard of practice; and maintenance of a clearinghouse for the collection and dis emination of scientific, tech nical.