19650003847.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446
Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION MEMORANDUM Date: March 11, 2020 SUBJECT: Registration Review Draft Risk Assessment for the Peroxy Compounds PC Code: 000595, 063201, 063604, 063607, DP Barcode: 455445, 455446 063209, 128860 Decision No: 558073, 558074 Docket No: EPA-HQ-OPP-2009-0546 Regulatory Action: Registration Review Case No: 6059, 4072, 5081 Risk Assessment Type: DRA CAS No: 7722-84-1, 79-21-0, 33734-57-5, 15630-89-4, 10058-23-8, 70693-62-8 TO: Kendall Ziner, Chemical Review Manager Rick Fehir, Ph.D., Team Lead Rose Kyprianou, Branch Chief Regulatory Management Branch (RMB) II Antimicrobials Division (7510P) Office of Pesticide Programs FROM: Andrew Byro, Ph.D., Chemist Kathryn Korthauer, Biologist Timothy Dole, Industrial Hygienist Deborah Burgin, Ph.D., DABT, Toxicologist Risk Assessment and Science Support Branch Antimicrobials Division (7510P) Office of Pesticide Programs THROUGH: Judy Facey, Ph.D., Human Health Risk Assessment Process Leader MP for JF Diana Hsieh, Ecological Risk Assessment Process Leader MP for DH Timothy Leighton, Senior Science Advisor MP for TL Laura Parsons, Associate Branch Chief Melissa Panger, Ph.D., Branch Chief Risk Assessment and Science Support Branch Antimicrobials Division (7510P) This document provides the draft human health and ecological risk assessment conducted in support of the antimicrobial use sites of the following peroxy compounds: hydrogen peroxide, peracetic acid, peroxyoctanoic acid, and sodium percarbonate. Page 1 of 74 Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 Although the peroxymonosulfate compounds were included in the peroxy compounds Final Work Plan (FWP), they will not be included in this risk assessment. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Appendix H EPA Hazardous Waste Law
Appendix H EPA Hazardous Waste Law This Appendix is intended to give you background information on hazardous waste laws and how they apply to you. For most U.S. Environmental Protection Agency (EPA) requirements that apply to the University, the Safety Department maintains compliance through internal inspections, record keeping and proper disposal. In Wisconsin, the Department of Natural Resources (DNR) has adopted the EPA regulations, consequently EPA and DNR regulations are nearly identical. EPA defines This Appendix only deals with "hazardous waste" as defined by the EPA. hazardous waste as Legally, EPA defines hazardous waste as certain hazardous chemical waste. This hazardous chemical Appendix does not address other types of regulated laboratory wastes, such as waste; radioactive, infectious, biological, radioactive or sharps. Chapter 8 descibes disposal procedures infectious and biohazardous waste for animals. Chapter 9 describes disposal procedures for sharps and other waste that are regulated by can puncture tissue. Chapter 11 discusses Radiation and the Radiation Safety for other agencies. Radiation Workers provides guidelines for the disposal of radioactive waste. Procedures for medical waste are written by the UW Hospital Safety Officer. The Office of Biological Safety can provide guidance for the disposal of infectious and biological waste. EPA regulations focus on industrial waste streams. As a result, many laboratory chemical wastes are not regulated by EPA as hazardous chemical waste. However, many unregulated chemical wastes do merit special handling and disposal If a waste can be procedures. Thus, Chapter 7 and Appendix A of this Guide recommend disposal defined as: procedures for many unregulated wastes as if they were EPA hazardous waste. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -

Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors
Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors by Derek A. Jackson A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Chemistry University of Toronto © Copyright by Derek A. Jackson 2013 Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors Derek Andrew Jackson Doctor of Philosophy Department of Chemistry University of Toronto 2013 Abstract This dissertation explores a number of different environmentally relevant reactions that lead to the production of perfluorocarboxylic acids (PFCAs), a family of environmental pollutants that does not undergo any further degradation pathways. The compound perfluoro-2-methyl-3-pentanone (PFMP) is a new fire fighting fluid developed by 3M that is designed as a Halon replacement. The environment fate of PFMP with regards to direct photolysis, abiotic hydrolysis and hydration was determined using a combination of laboratory experiments and computational modeling. PFMP was found to undergo direct photolysis giving a lifetime of 4-14 days depending on latitude and time of year. Offline samples confirmed PFCA products and a mechanism was proposed. Polyfluorinated amides (PFAMs) are a class of chemicals produced as byproducts of polyfluorinated sulfonamide synthesis via electrochemical fluorination (ECF). Using synthesized standards of four model compounds, PFAMs were detected and quantified in a variety of legacy commercial materials synthesized by ECF. PFAMs were hypothesized to undergo biological hydrolysis reactions, suggesting their importance as historical PFOA precursors. ii The PFAMs were also investigated with regards to their environmental fate upon atmospheric oxidation. Using a smog chamber, the kinetics and degradation mechanisms of N- ethylperfluorobutyramide (EtFBA) were elucidated. -
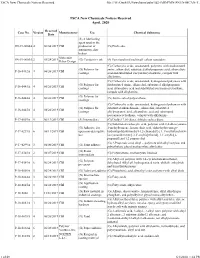
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

United States Patent 19 11 4,289,699 Oba Et Al
United States Patent 19 11 4,289,699 Oba et al. 45 Sep. 15, 1981 54 PROCESS FOR THE PRODUCTION OF Primary Examiner-Donald G. Daus N-(HYDROXYPHENYL) MALEIMEDES Assistant Examiner-D. B. Springer 75 Inventors: Masayuki Oba; Motoo Kawamata; Attorney, Agent, or Firm-Fisher, Christen & Sabol Hikotada Tsuboi; Nobuhito Koga, all 57 ABSTRACT of Yokohama, Japan N-(hydroxyphenyl) maleimides of the general formula 73 Assignee: Mitsui Toatsu Chemicals, Incorporated, Tokyo, Japan 21 Appl. No.: 88,825 (22 Filed: Oct. 26, 1979 N CO-m-CH Related U.S. Application Data (HO) 62) Division of Ser. No. 956,971, Nov. 2, 1978, Pat. No. 4,231,934. where R' stands for H, CH3, C2H5, F, Cl, Br or I and in 30 Foreign Application Priority Data is an integer of 1-5 are produced by treating the corre sponding maleamic acid or by treating the ester of said Nov. 2, 1977 (JP) Japan ................................ 52-130905 N-(hydroxyphenyl) maleimide at a temperature of Nov. 4, 1977 (JP Japan ................................ 52-3504 0-150° C. in the presence of at least one dehydrating 51) Int. Cl. .......................................... C07D 207/.452 agent selected for the group consisting of oxides and (52) U.S. Cl. .......................................... 260/326.5 FM oxyacids of sulfur or phosphorus and alkali metal and 58) Field of Search .............................. 260/326.5 FM alkaline earth metal salts of the said oxyacids. The cor responding maleamic acid can be obtained by reacting 56) - References Cited an aminophenol having one or more hydroxyl groups U.S. PATENT DOCUMENTS on its phenyl nucleus with maleic anhydride. -

WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/196440 Al 8 December 2016 (08.12.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61P 3/04 (2006.01) A61K 33/40 (2006.01) kind of national protection available): AE, AG, AL, AM, A61P 9/10 (2006.01) A61K 38/44 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 35/74 (2015.01) A61K 31/17 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/US20 16/034973 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 3 1 May 2016 (3 1.05.2016) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/169,480 1 June 2015 (01 .06.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/327,283 25 April 2016 (25.04.2016) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: XENO BIOSCIENCES INC. -

An Interstellar Synthesis of Phosphorus Oxoacids
ARTICLE DOI: 10.1038/s41467-018-06415-7 OPEN An interstellar synthesis of phosphorus oxoacids Andrew M. Turner1,2, Alexandre Bergantini 1,2, Matthew J. Abplanalp1,2, Cheng Zhu 1,2, Sándor Góbi 1,2, Bing-Jian Sun3, Kang-Heng Chao3, Agnes H.H. Chang3, Cornelia Meinert 4 & Ralf I. Kaiser 1,2 Phosphorus signifies an essential element in molecular biology, yet given the limited solubility of phosphates on early Earth, alternative sources like meteoritic phosphides have been proposed to incorporate phosphorus into biomolecules under prebiotic terrestrial conditions. Here, we report on a previously overlooked source of prebiotic phosphorus from interstellar 1234567890():,; phosphine (PH3) that produces key phosphorus oxoacids—phosphoric acid (H3PO4), phos- phonic acid (H3PO3), and pyrophosphoric acid (H4P2O7)—in interstellar analog ices exposed to ionizing radiation at temperatures as low as 5 K. Since the processed material of molecular clouds eventually enters circumstellar disks and is partially incorporated into planetesimals like proto Earth, an understanding of the facile synthesis of oxoacids is essential to untangle the origin of water-soluble prebiotic phosphorus compounds and how they might have been incorporated into organisms not only on Earth, but potentially in our universe as well. 1 Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA. 2 W.M. Keck Laboratory in Astrochemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA. 3 Department of Chemistry, National Dong Hwa University, Shoufeng, 974 Hualien, Taiwan. 4 Université Côte d’Azur, CNRS, Institut de Chimie de Nice, Nice, France. Correspondence and requests for materials should be addressed to R.I.K. -
Us 2012/0074.014 A1 2 .. 1
US 2012O074O14A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0074.014 A1 Tran et al. (43) Pub. Date: Mar. 29, 2012 (54) PRODUCT TYPICALLY BASED ON SALT OF Publication Classification PEROXYMONOSULFURIC ACID AND (51) Int. Cl SUITABLE FOR MEDICINAL USAGE, AND iBio/02 (2006.01) ASSOCATED PRODUCT FABRICATION B23P 19/00 (2006.01) A633/42 (2006.01) (75) Inventors: David Van Tran, San Jose, CA A6IR 9/14 (2006.01) (US); David Nguyen Tran, San CD7C 409/244 (2006.01) Jose, CA (US) A6II 3/327 (2006.01) s (52) U.S. Cl. ............. 206/438: 562/1; 514/578; 424/605; 424/400; 29/428 (73) Assignee: LuTran Industries, Inc. (57) ABSTRACT 21) Appl. No.: 13AO47,742 Products based on salt of peroxyperoxVmonosulfuric acid are suit (21) Appl. No 9 able for treating or/and preventing diseases and other debili tating medical conditions caused by bacterial, eukaryotic, (22) Filed: Mar. 14, 2011 prion, and viral pathogens and by non-pathogenic inflamma tion. The products may alternatively be based on inorganic Related U.S. Application Data halide and an oxidizing agent reactable in water with the inorganic halide to generate hypohalite ions. In addition, the (60) Provisional application No. 61/386,928, filed on Sep. products can be employed in other applications such as com 27, 2010. mercial and industrial applications. 5T. 2 .. 2. 1.4. J., Patent Application Publication Mar. 29, 2012 Sheet 1 of 2 US 2012/0074014 A1 cro Q-D 22 Fig. 2a Fig.2b Patent Application Publication Mar. 29, 2012 Sheet 2 of 2 US 2012/0074014 A1 90 92 US 2012/0074014 A1 Mar.