SAFETY DATA SHEET Sodium Persulfate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Cover Page for Safety Data Sheet
Simplicity in Water Analysis Cover Page for Safety Data Sheet Thank you for choosing CHEMetrics, Inc. We appreciate your business. In order to best serve your needs for accurate and complete Safety Data, we offer the following information as supplemental to the attached SDS. SDS No.: S4201 Version No.: 2.2 Product Name: Activator Solution for Formaldehyde CHEMets®, VACUettes®, & Vacu-vials® Kits, and for Glycol CHEMets® & Vacu-vial® Kits Part Nos.: A-4201, A-4401 Product Descriptions: Activator Solution: Plastic bottle, contains approximately 3.4 g of solid reagent. Test kits contain one (1) bottle of reagent. Activator Solution packs contain six (6) bottles of reagent. Addendum to Section 14 Transport Information: Shipping container markings and labels for this product, as received, may vary from the contents of section 14 of the SDS for one or both of the following reasons: • CHEMetrics has packaged this product as Dangerous Goods in Excepted Quantities according to IATA, US DOT, and IMDG regulations. • CHEMetrics has packaged this product as part of a test kit or reagent set composed of various chemical reagents and elected to ship as UN 3316 Chemical Kit, Hazard Class 9, Packing Group II or III. In case of reshipment, it is the responsibility of the shipper to determine appropriate labels and markings in accordance with applicable transportation regulations. Additional Information: • “Print Date” = Revision Date (expressed as DD/MM/YYYY) • Test kits and reagents sets may contain additional chemical reagents. See separate SDS(s). CHEMets®, VACUettes®, Vacu-vials®, and Titrets® are registered trademarks of CHEMetrics Inc. 4295 Catlett Road, Midland, VA 22728 P: 800.356.3072 F: 540.788.4856 www.chemetrics.com Activator Solution for Formaldehyde CHEMets, VACUettes, & Vacu-vials Kits, and for Glycol CHEMets & Vacu-vials Kits CHEMetrics, Inc. -

Safety Data Sheet: Sodium Persulfate
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH), amended by 2015/830/EU Sodium persulfate ≥ 99% article number: 4365 date of compilation: 2016-07-05 Version: 2.0 en Revision: 2019-11-27 Replaces version of: 2016-07-05 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Identification of the substance Sodium persulfate Article number 4365 Registration number (REACH) 01-2119495975-15-xxxx EC number 231-892-1 CAS number 7775-27-1 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: laboratory chemical laboratory and analytical use 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone: +49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data : Department Health, Safety and Environment sheet e-mail (competent person) : [email protected] 1.4 Emergency telephone number Name Street Postal code/city Telephone Website National Poisons In- Dudley Rd B187QH Birmingham 844 892 0111 formation Service City Hospital Emergency information service +49/(0)89 19240 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Classification acc. to GHS Section Hazard class Hazard class and cat- Hazard egory state- ment 2.14 oxidising solid (Ox. Sol. 3) H272 3.1O acute toxicity (oral) (Acute Tox. 4) H302 United Kingdom (en) Page 1 / 16 Safety data sheet according to Regulation (EC) No. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -
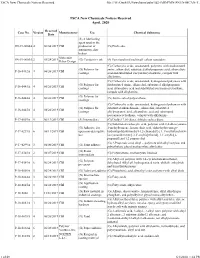
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

Reactivo Catalogue Copy
Reactivo® PRODUCT CATALOGUES 2020 - 2021 Traceable Chemicals | Reagents | Standards | Buffers For laboratories and productions Designed for Quality Team Reactivo® is proud to introduce our latest range of reagents manufactured in Singapore. Reactivo®’s slogan is “Designed for Quality” , which emphasize on the best quality, and unique capability of providing personalised service. Our products have passed stringent quality testing throughout the production process, which further prevents contamination before sealing and bottling. Packaging info packaging is very essential to the product that we deliver. Available icon 100 ml 500 ml 1000 ml 2000 ml 5000 ml 10000 ml 25000 ml IBC 100000 ml P HDPE Bottle P HDPE Bottle P HDPE Bottle P HDPE Bottle P HDPE Jerry can P HDPE Carboy P HDPE Carboy IBC Tank 500 ml 1000 ml 4000 ml G Glass Bottle G Glass Bottle G Glass Bottle Catalogue directory Guide and legend on how to read the catalogue, and it’s content A Sodium Persulfate (Sodium peroxodisulphate) Legends It is mainly used as a radical initiator for emulsion polymerization reactions for A Title B styrene based polymers such as Acrylonitrile butadiene styrene. G B Application CAS No. Molar Mass 7775-27-1 238.10 g/mol G C Chemical structure C Chemical Formula IUPAC Name D Order info Na S O Sodium peroxydisulfate 2 2 8 E Concentration F Packaging options Product No. Concentration Packaging G GHS Pictograms RSIT001 0.05 N 100 ml 500 ml 1000 ml 1000 ml 4000 ml D Standardized P HDPE Bottle P HDPE Bottle P HDPE Bottle G Glass Bottle G Glass Bottle E F Label directory Guide and legend on how to read the product label, and it’s content Legends 1 Item No. -

Revision Date: March 2021 1 SODIUM PERSULFATE This Dossier On
SODIUM PERSULFATE This dossier on sodium persulfate presents the most critical studies pertinent to the risk assessment of sodium persulfate in its use in hydraulic fracturing fluids. This dossier does not represent an exhaustive or critical review of all available data. Where possible, study quality was evaluated using the Klimisch scoring system (Klimisch et al., 1997). Screening Assessment Conclusion – Sodium persulfate is classified as a tier 1 chemical and requires a hazard assessment only. 1 BACKGROUND Sodium persulfate dissociates in aqueous media to the sodium cation (Na+) and persulfate anion 2- (S2O8 ). The persulfate anion will readily hydrolyse (decompose) into sulfate ions. Biodegradation is not applicable to inorganic compounds. Sodium persulfate is not expected to bioaccumulate; it will dissociate (and decompose) to ions that are ubiquitous in the environment. Sodium persulfate is not expected to absorb to soil or sediment because of its dissociation properties, instability (hydrolysis) and high water solubility. Sodium persulfate exhibits moderate acute toxicity by the oral route and low acute toxicity by the inhalation and dermal routes. In humans, sodium persulfate has the potential for skin irritation; it is also a skin sensitiser to guinea pigs and humans. Human exposure to persulfates (including sodium persulfate) have been linked to a variety of skin and respiratory complaints indicative of sensitisation. The complaints consist of immediate and delayed contact hypersensitivity, contact urticarial, rhinitis, bronchitis and asthma. Repeated oral exposure to sodium persulfate resulted in irritation to the gastrointestinal tract; and respiratory irritation was seen in rats repeatedly exposed by inhalation to ammonium persulfate. Sodium persulfate is not genotoxic. -

Application Note 06
Application Note 06 Synthesis StarterKit Production of Persulfate Description This Application Note describes the oxidative transfor- mation of sodium sulfate to sodium persulfate by using a BDD electrode. The setup is depicted in Figure 1. The following chemical equation is summing up all reactants and products: 2 Na2SO4 + 2 H2O → Na2S2O8 + 2 NaOH + H2 The oxidation number of the sulfate atoms is staying constant while the oxidation number of two oxygen at- oms if changing from -II to –I in the peroxo group. Experiment Figure 1: For the experiment, 1000 ml of a 1 mol/l sodium sulfate Setup to produce persulfate solution is prepared in beaker glass suitable to be used with the Synthesis StarterKit. The volume flow is set to 2 l/min. The electrosynthesis is carried out at 1.3 A which corresponds to a current density of j = 0.4 A/cm². Table 1 shows the experimental data collected during the experiment. Table 1: Experimental data obtained during the experiment Charge Time Current Voltage Temp. V(Thio) c(Ox) [Ah/L] [min.] [A] [V] [°C] [ml] [mmol/l] 0 0 1.3 5.6 20.5 --- --- 0.22 10 1.3 5.7 23.0 0.90 2.15 0.44 20 1.3 5.7 26.0 1.55 3.60 0.66 30 1.3 5.7 28.0 2.30 5.16 0.89 40 1.3 5.7 30.0 2.80 6.14 1.13 50 1.3 5.6 32.0 3.40 7.26 1.37 60 1.3 5.6 33.5 4.00 8.33 The concentration of oxidizer was determined by iodometry ( see appendix and App Note 07) www.condias.de Application Note 06 Synthesis StarterKit Production of Persulfate Figure 2: Graphical representation of the evolution of persulfate concentration In Figure 2 the graphical representation if given for the evolution of the persulfate concentration as a function of charge. -

B COMMISSION DELEGATED REGULATION (EU) No
02014R1062 — EN — 21.02.2019 — 002.001 — 1 This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document ►B COMMISSION DELEGATED REGULATION (EU) No 1062/2014 of 4 August 2014 on the work programme for the systematic examination of all existing active substances contained in biocidal products referred to in Regulation (EU) No 528/2012 of the European Parliament and of the Council (Text with EEA relevance) (OJ L 294, 10.10.2014, p. 1) Amended by: Official Journal No page date ►M1 Commission Delegated Regulation (EU) 2017/698 of 3 February 2017 L 103 1 19.4.2017 ►M2 Commission Delegated Regulation (EU) 2019/157 of 6 November 2018 L 31 1 1.2.2019 Corrected by: ►C1 Corrigendum, OJ L 198, 28.7.2015, p. 28 (1062/2014) 02014R1062 — EN — 21.02.2019 — 002.001 — 2 ▼B COMMISSION DELEGATED REGULATION (EU) No 1062/2014 of 4 August 2014 on the work programme for the systematic examination of all existing active substances contained in biocidal products referred to in Regulation (EU) No 528/2012 of the European Parliament and of the Council (Text with EEA relevance) CHAPTER 1 SUBJECT MATTER AND DEFINITIONS Article 1 Subject matter This Regulation lays down rules for the carrying out of the work programme for the systematic examination of all existing active substances referred to in Article 89 of Regulation (EU) No 528/2012. -

Chemical Compatibility Storage Group
CHEMICAL SEGREGATION Chemicals are to be segregated into 11 different categories depending on the compatibility of that chemical with other chemicals The Storage Groups are as follows: Group A – Compatible Organic Acids Group B – Compatible Pyrophoric & Water Reactive Materials Group C – Compatible Inorganic Bases Group D – Compatible Organic Acids Group E – Compatible Oxidizers including Peroxides Group F– Compatible Inorganic Acids not including Oxidizers or Combustible Group G – Not Intrinsically Reactive or Flammable or Combustible Group J* – Poison Compressed Gases Group K* – Compatible Explosive or other highly Unstable Material Group L – Non-Reactive Flammable and Combustible, including solvents Group X* – Incompatible with ALL other storage groups The following is a list of chemicals and their compatibility storage codes. This is not a complete list of chemicals, but is provided to give examples of each storage group: Storage Group A 94‐75‐7 2,4‐D (2,4‐Dichlorophenoxyacetic acid) 94‐82‐6 2,4‐DB 609-99-4 3,5-Dinitrosalicylic acid 64‐19‐7 Acetic acid (Flammable liquid @ 102°F avoid alcohols, Amines, ox agents see SDS) 631-61-8 Acetic acid, Ammonium salt (Ammonium acetate) 108-24-7 Acetic anhydride (Flammable liquid @102°F avoid alcohols see SDS) 79‐10‐7 Acrylic acid Peroxide Former 65‐85‐0 Benzoic acid 98‐07‐7 Benzotrichloride 98‐88‐4 Benzoyl chloride 107-92-6 Butyric Acid 115‐28‐6 Chlorendic acid 79‐11‐8 Chloroacetic acid 627‐11‐2 Chloroethyl chloroformate 77‐92‐9 Citric acid 5949-29-1 Citric acid monohydrate 57-00-1 Creatine 20624-25-3 -

19650003847.Pdf
NATIONAL BUREAU OF STANDARDS REPO RT 8595 PRELIMINARY REPORT ON A SURVEY OF THERMODYNAMIC PROPERTIES OF THE COMPOUNDS OF THE ELEMENTS CHNOPS i N65 2 CACC£S$IO NUMB£:R) CTHRU) ~ ___f£ / I- (PAGES) (CdOEI ::; ~ CIU - d -P?02c2/ :33 (NASA CR OR TMX OR AD NUMBER) (CATEQORY) Progress Report for the Period 1 August to 31 October 1964 to National Aeronautics and Space Administration GPO PRICE $ _____ OTS PRICE(S) $ 1 November 1964 Hard copy (He) of· cJV r Microfiche (MF) __....;... : .:::.j~I?J~_ <@> U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARD S The ational Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. It responsibilities include development and main tena nce of the national stand· ards of measurement, and the provisions of means for making measurements consi tent with those standard ; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be nece sary, particu larl: for ~overn ment agencies; cooperation in the establi hment of standard practices for incorpora tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of device to serve special needs of the Government: assi tance to indus! r). business. and consumers in the development and acceptance of commercial tandard and simplified trade practice recommendations; administration of programs in cooperation with United tates bu iness groups and standards organizations for the development of international standard of practice; and maintenance of a clearinghouse for the collection and dis emination of scientific, tech nical.