The Following List Contains the Material Safety Data Sheets You Requested
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Acrylamide Polymerization — a Practical Approach
electrophoresis tech note 1156 Acrylamide Polymerization — A Practical Approach Paul Menter, Bio-Rad Laboratories, 2000 Alfred Nobel Drive, Polyacrylamide Gel Polymerization Hercules, CA 94547 USA AcrylamideBis Polyacrylamide Introduction The unparalleled resolution and flexibility possible with CH2 CH + CH2 CH CH2 CH CH2 CH CH2 CH polyacrylamide gel electrophoresis (PAGE) has led to its CO CO CO CO CO widespread use for the separation of proteins and nucleic NH2 NH NH2 NH2 NH acids. Gel porosity can be varied over a wide range to meet CH2 CH2 specific separation requirements. Electrophoresis gels and NH NH NH NH buffers can be chosen to provide separation on the basis of CO 2 2 CO CO C O charge, size, or a combination of charge and size. CH2 CH CH2 CH CH2 CH CH2 CH The key to mastering this powerful technique lies in the polymerization process itself. By understanding the important Purity of Gel-Forming Reagents parameters, and following a few simple guidelines, the novice Acrylamide can become proficient and the experienced user can optimize Gel-forming reagents include the monomers, acrylamide and bis, separations even further. as well as the initiators, usually ammonium persulfate and TEMED or, occasionally, riboflavin and TEMED. On a molar This bulletin takes a practical approach to the preparation of basis, acrylamide is by far the most abundant component in the polyacrylamide gels. Its purpose is to provide the information monomer solution. As a result, acrylamide may be the primary required to achieve reproducible, controllable polymerization. source of interfering contaminants (Dirksen and Chrambach For those users interested only in the “bare essentials,” the 1972). -

(Ph.D.) Environmental Engineering
UNIVERSITY OF CINCINNATI Date: June 15, 2005 I, Georgios Anipsitakis , hereby submit this work as part of the requirements for the degree of: Doctorate of Philosophy (Ph.D.) in: Environmental Engineering It is entitled: Cobalt/Peroxymonosulfate and Related Oxidizing Reagents for Water Treatment This work and its defense approved by: Chair: Dr. Dionysios Dionysiou Dr. Paul Bishop Dr. George Sorial Dr. Souhail Al-Abed COBALT/PEROXYMONOSULFATE AND RELATED OXIDIZING REAGENTS FOR WATER TREATMENT A dissertation submitted to the Division of Research and Advanced Studies of the University of Cincinnati in partial fulfillment of the requirements for the degree of DOCTORATE OF PHILOSOPHY (Ph.D.) in the Department of Civil and Environmental Engineering of the College of Engineering 2005 by Georgios P. Anipsitakis Diploma (B.S./M.S.), Chemical Engineering, Nat. Technical Univ. Athens, 2000 Committee Chair: Dr. Dionysios D. Dionysiou ii Abstract This dissertation explores the fundamentals of a novel advanced oxidation technology, the II cobalt/peroxymonosulfate (Co /KHSO5) reagent, for the treatment of persistent and hazardous II substances in water. Co /KHSO5 is based on the chemistry of the Fenton Reagent and proceeds via the generation of sulfate radicals, which similarly to hydroxyl radicals, readily attack and degrade organic and microbial contamination in water. Very few studies have exploited the reactivity of sulfate radicals for environmental applications. Compared to the extensively investigated hydroxyl radicals, sulfate radicals are not fully understood. Following this approach, the coupling of nine transition metals with hydrogen peroxide (H2O2), potassium peroxymonosulfate (KHSO5) and persulfate (K2S2O8) was also explored. The objective was again the generation of inorganic radicals and the efficient degradation of organic contaminants in water. -

Microchip Design for Chemiluminesence Detection
Double spiral detection channel for on-chip chemiluminescence detection Author Lok, Khoi Seng, Kwok, Yien Chian, Nam-Trung, Nguyen Published 2012 Journal Title Sensors and Actuators B: Chemical DOI https://doi.org/10.1016/j.snb.2012.04.047 Copyright Statement © 2012 Elsevier B.V.. This is the author-manuscript version of this paper. Reproduced in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/50085 Griffith Research Online https://research-repository.griffith.edu.au Lab on a Chip for Chemiluminescence Detection Khoi Seng Lok1, Yien Chian Kwok1* and Nam Trung Nguyen2 1National Institute of Education, Nanyang Technological University, Nanyang Walk, Singapore 637616, Singapore. E-mail: [email protected] 2School of Mechanical and Production Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore. E-mail: [email protected] Abstracts In this paper reports a three layered device that consists of a double spiral channel design for chemiluminsence (CL) detection and a passive micromixer to facilitate mixing of reagents. Compared to the design with a single spiral channel, the design with two overlapping spiral channels doubled the CL intensity emitted from the reaction. Furthermore, the addition of the passive micromixer improved the signal by 1.5 times. Luminol-based chemiluminescence reaction was used for the characterization of the device. This device was successfully used for the determination of L-cysteine and uric acid. Keywords: chemiluminescence, lab-on-chip, micro total analysis system, luminol, cobalt, L-cysteine, uric acid 1 Introduction Chemiluminescence (CL) detection methods do not require an excitation light source. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -
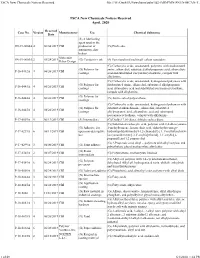
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways
International Journal of Molecular Sciences Review ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways Igor R. Ilyasov *, Vladimir L. Beloborodov, Irina A. Selivanova and Roman P. Terekhov Department of Chemistry, Sechenov First Moscow State Medical University, Trubetskaya Str. 8/2, 119991 Moscow, Russia; [email protected] (V.L.B.); [email protected] (I.A.S.); [email protected] (R.P.T.) * Correspondence: [email protected]; Tel.: +7-985-764-0744 Received: 30 November 2019; Accepted: 5 February 2020; Published: 8 February 2020 + Abstract: The 2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS• ) radical cation-based assays are among the most abundant antioxidant capacity assays, together with the 2,2-diphenyl-1- picrylhydrazyl (DPPH) radical-based assays according to the Scopus citation rates. The main objective of this review was to elucidate the reaction pathways that underlie the ABTS/potassium persulfate decolorization assay of antioxidant capacity. Comparative analysis of the literature data showed that there are two principal reaction pathways. Some antioxidants, at least of phenolic nature, + can form coupling adducts with ABTS• , whereas others can undergo oxidation without coupling, thus the coupling is a specific reaction for certain antioxidants. These coupling adducts can undergo further oxidative degradation, leading to hydrazindyilidene-like and/or imine-like adducts with 3-ethyl-2-oxo-1,3-benzothiazoline-6-sulfonate and 3-ethyl-2-imino-1,3-benzothiazoline-6-sulfonate as marker compounds, respectively. The extent to which the coupling reaction contributes to the total antioxidant capacity, as well as the specificity and relevance of oxidation products, requires further in-depth elucidation. -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Safety Data Sheet
World Headquarters Page 1 Hach Company Date Printed 10/25/15 P.O.Box 389 MSDS No: M00039 Loveland, CO USA 80539 (970) 669-3050 SAFETY DATA SHEET _____________________________________________________________________________ 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Potassium Persulfate Catalog Number: 2084769 Hach Company Emergency Telephone Numbers: P.O.Box 389 (Medical and Transportation) Loveland, CO USA 80539 (303) 623-5716 24 Hour Service (970) 669-3050 (515)232-2533 8am - 4pm CST MSDS Number: M00039 Chemical Name: Peroxydisulfuric Acid, Dipotassium Salt CAS Number: 7727-21-1 Additional CAS No. (for hydrated forms): Not applicable Chemical Formula: K2S2O8 Chemical Family: Oxidizing Agents Intended Use: Laboratory Use _____________________________________________________________________________ 2. HAZARDS IDENTIFICATION GHS Classification: Hazard categories: Oxidizing Solids: Ox. Sol. 3 Acute Toxicity: Acute Tox. 4-Orl Skin Corrosion/Irritation: Skin Irrit. 2 Respiratory or Skin Sensitization: Skin Sens.1 Serious Eye Damage/Eye Irritation:Eye Irrit. 2 Respiratory or Skin Sensitization: Resp. Sens.1 Specific Target Organ Toxicity - Single Exposure: STOT SE 3 GHS Label Elements: DANGER Hazard statements: May intensify fire; oxidiser. Harmful if swallowed. Causes skin irritation. May cause an allergic skin reaction. Causes serious eye irritation. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause respiratory irritation. Precautionary statements: Wear protective gloves / protective clothing / eye protection / face protection. Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Keep/Store away from clothing/combustible materials. Avoid breathing dust/fume/gas/mist/vapours/spray. Use only outdoors or in a well-ventilated area. Wear respiratory protection. Do no eat, drink or smoke when using this product. -

SAFETY DATA SHEET Sodium Persulfate
SAFETY DATA SHEET Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Format: NA Version 1.06 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name Sodium Persulfate CAS-No 7775-27-1 Synonyms Sodium Peroxydisulfate; Disodium Peroxydisulfate; Peroxydisulfuric acid, disodium salt; Peroxydisulfuric acid, sodium salt. Recommended use of the chemical and restrictions on use Recommended Use: Polymerization initiator; Etchant and cleaner for printed circuit boards; Hair bleaching formulations; Secondary oil recovery; Oxidizing agent for a variety of organic reactions. Restrictions on Use No uses to be advised against were identified. Manufacturer/Supplier PeroxyChem LLC 2005 Market Street Suite 3200 Philadelphia, PA 19103 Phone: +1 267/ 422-2400 (General Information) E-Mail: [email protected] Emergency telephone numbers For leak, fire, spill or accident emergencies, call: 1 800 / 424 9300 (CHEMTREC - U.S.A.) 1 703 / 527 3887 (CHEMTREC - Collect - All Other Countries) +1 303/ 389-1409 (Medical - U.S. - Call Collect) Page 1 / 10 Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Version 1.06 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2B Respiratory sensitization Category 1 Skin sensitization Category 1 Specific target organ toxicity (single exposure) Category 3 Oxidizing Solids -

United States Patent Office Patented July 6, 1965 2 Produces an Exothermic Chemical Reaction
3,193,464 United States Patent Office Patented July 6, 1965 2 produces an exothermic chemical reaction. This heat 3,193,464 intensifies the sensitivity of the scalp to the peroxide HYDROGEN PEROXDE HAR BLEACHING free alkali, etc. COMPOSITION AND METHOD Hair color consists of granular coloring such as black, Walter W. Edman, Port Washington, and Anne T. Sulli 5 which is easily bleached out, and diffused red coloring van, Hollis, N.Y., assignors to Sales Affilites, Inc., New which is more resistant to bleaching. Because of its re York, N.Y., a corporation of New York sistance, red tones are found in all but the higher stages No Drawing. Filed May 31, 1961, Ser. No. 113,605 of bleached hair. The shades and intensity of red in 5 Claims. (C. 167-88) the hair vary from individual to individual and even O on the same head. As the principal purpose of bleaching This invention relates to a novel hair bleaching com is to arrive at a blonde shade, these red tones produce position and particularly to a hair bleaching composi an undesirable color effect. They confer a highly arti tion that produces a more effective, more versatile and ficial-looking color to hair and impart a brassy tone to more comfortable bleaching action on the hair. blonde shades. Any red hue remaining in the hair inter Any process relating to the treatment of hair must take 5 feres with the color imparted by the hair toner, producing into account a tremendous number of variables; the an off-color. The presently marketed bleaches eliminate bleaching process is no exception.