Ponds of the Phoenix Park Ecological Status and Future Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

'Dublin's North Inner City, Preservationism and Irish Modernity in the 1960S'
Edinburgh Research Explorer Dublin’s North Inner City, Preservationism and Irish Modernity in the 1960s Citation for published version: Hanna, E 2010, 'Dublin’s North Inner City, Preservationism and Irish Modernity in the 1960s', Historical Journal, vol. 53, no. 4, pp. 1015-1035. https://doi.org/10.1017/S0018246X10000464 Digital Object Identifier (DOI): 10.1017/S0018246X10000464 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Historical Journal Publisher Rights Statement: © Hanna, E. (2010). Dublin’s North Inner City, Preservationism and Irish Modernity in the 1960s. Historical Journal, 53(4), 1015-1035doi: 10.1017/S0018246X10000464 General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 28. Sep. 2021 The Historical Journal http://journals.cambridge.org/HIS Additional services for The Historical Journal: Email alerts: Click here Subscriptions: Click here Commercial reprints: Click here Terms of use : Click here DUBLIN'S NORTH INNER CITY, PRESERVATIONISM, AND IRISH MODERNITY IN THE 1960S ERIKA HANNA The Historical Journal / Volume 53 / Issue 04 / December 2010, pp 1015 - 1035 DOI: 10.1017/S0018246X10000464, Published online: 03 November 2010 Link to this article: http://journals.cambridge.org/abstract_S0018246X10000464 How to cite this article: ERIKA HANNA (2010). -

PHOENIX PARK TRANSPORT and MOBILITY OPTIONS STUDY Public Consultation Brochure | January 2021 PROJECT DESCRIPTION the PREFERRED OPTION
PHOENIX PARK TRANSPORT AND MOBILITY OPTIONS STUDY Public Consultation Brochure | January 2021 PROJECT DESCRIPTION THE PREFERRED OPTION The Office of Public Works with the National The Phoenix Park Transport and Mobility J Traffic will be reduced on the North Road and Transport Authority, Dublin City Council Options Report makes a number of key the Upper Glen Road so as to improve the and Fingal County Council, at a request recommendations including the following: amenities in these areas. In the medium to long of Minister of State at the Office of Public term, vehicular restriction will be introduced at Works, developed a framework to help J Prioritise pedestrian infrastructure Cabra, Ashtown and Knockmaroon Gates. shape and inform a vision for how visitors including the upgrade of over 7km of will access, experience and move within the footpaths along with strategic pedestrian J In the short to medium term a bus service will be Phoenix Park while protecting its character crossing points on Chesterfield Avenue and introduced for Dublin Zoo and the Phoenix Park and biodiversity, and thus enhancing the other key locations throughout the Park, Visitor Centre, serving all areas along this route overall visitor experience. including the Gate entrances. and linking to Heuston Station and Broombridge Luas Station. J Expand and upgrade the cycle network The Report is based on a set of core within the Park and linkages to the external J The speed limit will be set at 30kph with a review Movement Principles; that the Park is for networks to facilitate all cycling users. This of parking and byelaws being recommended. -

Draft Fingal County Development Plan 2017-2023
DRAFT FINGAL COUNTY DEVELOPMENT PLAN 2017-2023 SUBMISSION RELATING TO FORMER PHOENIX PARK RACECOURSE & ADJOINING LANDS, CASTLEKNOCK, DUBLIN 15 PART A: FORMER RACECOURSE - LANDS SOUTH OF THE N3 PART B: RAILWAY SITE - LANDS NORTH OF THE N3 On behalf of: FLYNN & O’FLAHERTY CONSTRUCTION April 2016 1.0 INTRODUCTION 1.1 Purpose of Submission On behalf of Flynn & O’Flaherty Construction (hereafter F&OF) the following submission to the proposed Draft Fingal County Development Plan 2017-2023 is made in respect of the former Phoenix Park Racecourse and adjoining lands, Castleknock, Dublin 15. The purpose of the current submission is to identify the policies and objectives of the Draft Fingal Development Plan that relate to the F&FO’F lands at the former Phoenix Park Racecourse and north of the N3 and to suggest “Amendments” that will facilitate the ongoing development of the lands over period 2017-2023 and beyond. 1.2 Lands Subject of this Submission Figure 1 identifies the location and extent of the F&OF lands. The overall F&OF land holding comprises 42.8ha. The F&O’F lands are described in detail within Section 2.0 to 4.0 below. However, for clarity and to reflect their very different planning considerations, this submission divides the lands into two parts as follows: - PART A: Former Phoenix Park Racecourse – Lands south of the N3 - 37.9ha. PART B: Railway Site – Lands north of the N3 – 4.9ha. (This area is part of the lands referred to in the Draft Development Plan as the “Navan Road Parkway” Local Area Plan lands). -

Negotiating Ireland – Some Notes for Interns
Welcome to Ireland – General Notes for Interns (2015 – will be updated for 2016 in January 2016) Fergus Ryan These notes are designed to introduce you to Ireland and to address any questions you might have concerning practical aspects about your visit to Ireland. About Ireland Ireland is an island on the north- financial services. The official west coast of Europe, with a languages are English and Irish. population of approximately 6.3 While English is the main language million inhabitants. It is of communication, Irish is spoken on approximately 32,600 square miles, a daily basis in some parts of the 300 miles from the northern most west, while over half a million tip to the most southern, and inhabitants speak a language other approximately 175 miles across, than English or Irish at home. making it just a little under half the (Sources: CSO Census 2011, size of Oklahoma State. www.cso.ie) Politically, the island comprises two Northern Ireland comprises six legal entities. The Republic of counties in the northeast corner of Ireland, with 4.6 million the island. A jurisdiction within the inhabitants, makes up the bulk of the United Kingdom, it has just over 1.8 island. The State attained million people. It has its own power- independence from the UK in 1922, sharing parliament and government and became a Republic in 1949. The with significant devolved powers Republic of Ireland is a sovereign, and functions. Its capital and largest democratic republic, with its current city is Belfast. Northern Ireland is Constitution dating back to 1937. It politically divided along religious is a member of the European Union lines: 48% of those in Northern and the Council of Europe, but is Ireland are Protestant or were militarily non-aligned. -

66 Bus Time Schedule & Line Route
66 bus time schedule & line map 66 Merrion Square South - Kingsbury Estate View In Website Mode The 66 bus line (Merrion Square South - Kingsbury Estate) has 2 routes. For regular weekdays, their operation hours are: (1) Merrion Square South - Kingsbury Estate: 6:00 AM - 11:15 PM (2) Straffan Road (Kingsbury Estate) - Merrion Square South: 5:45 AM - 11:15 PM Use the Moovit App to ƒnd the closest 66 bus station near you and ƒnd out when is the next 66 bus arriving. Direction: Merrion Square South - Kingsbury 66 bus Time Schedule Estate Merrion Square South - Kingsbury Estate Route 60 stops Timetable: VIEW LINE SCHEDULE Sunday 7:05 AM - 11:05 PM Monday 6:00 AM - 11:15 PM Merrion Sq South, Stop 7391 Merrion Square South, Dublin Tuesday 6:00 AM - 11:15 PM Holles Street, Stop 493 Wednesday 6:00 AM - 11:15 PM 27 Merrion Square North, Dublin Thursday 6:00 AM - 11:15 PM Clare Street Friday 6:00 AM - 11:15 PM 20 Clare Street, Dublin Saturday 6:15 AM - 11:15 PM Pearse Station, Stop 495 Westland Row, Dublin Shaw Street, Stop 400 194 Pearse Street, Dublin 66 bus Info Direction: Merrion Square South - Kingsbury Estate Pearse St Garda Stn, Stop 346 Stops: 60 17 Botany Bay, Dublin Trip Duration: 68 min Line Summary: Merrion Sq South, Stop 7391, Holles Westmoreland Street Street, Stop 493, Clare Street, Pearse Station, Stop 28 Westmoreland Street, Dublin 495, Shaw Street, Stop 400, Pearse St Garda Stn, Stop 346, Westmoreland Street, Temple Bar, Temple Bar, Wellington Quay Wellington Quay, Merchant's Quay, Stop 1444, 11 Essex Street East, Dublin Usher's -

The Phoenix Park Visitor Guide
Recreation Beautiful vistas of the Dublin Mountains, tree lined paths, wildflower meadows, Phoenix Park Phoenix Park - A National Historic Park Over 2,300 sporting events take place in the lakeside and woodland walks are just some Phoenix Park in the intensive recreation of the many opportunities to enjoy in the The Phoenix Park at 707 hectares is one of the largest enclosed zone every year. They are organised by park. Parks within any European City. accredited sporting organisations to train VISITOR’S GUIDE and play matches such as soccer, gaelic Biodiversity SIGNIFICANT PARK DATES football, hurling and camogie.There are also General demesne of Kilmainham Priory south of the many athletic events. About 30% of the Phoenix Park is covered c.1177 Hugh Tyrell 1st Baron of Castleknock, granted River Liffey, but with the building of the A wide range of recreational activities by trees, which are mainly broadleaf The Phoenix Park at 707 hectares is one Royal Hospital at Kilmainham, which including orienteering, astronomy, cross land, including what is now Phoenix Park land, parkland species such as oak, ash, lime, of the largest enclosed recreational spaces (commenced in 1680), the Park was country events, athletics, cycle races and beech, sycamore and horsechestnut. A to the Knights of St. John of Jerusalem at within any European capital city. It is larger reduced to its present size, all of which is model aeroplane flying are organised by more ornamental selection of trees is Kilmainham. than all of London’s city parks put together, now North of the river Liffey. In 1747 the various clubs and groups who use the park. -
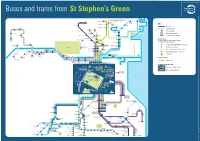
Buses and Trams from St Stephen's Green
142 Buses and trams from St Stephen’s Green 142 continues to Waterside, Seabury, Malahide, 32x continues to 41x Broomfield, Hazelbrook, Sainthelens and 15 Portmarnock, Swords Manor Portmarnock Sand’s Hotel Baldoyle Malahide and 142 Poppintree 140 Clongriffin Seabury Barrysparks Finglas IKEA KEY Charlestown SWORDS Main Street Ellenfield Park Darndale Beaumont Bus route Fosterstown (Boroimhe) Collinstown 14 Coolock North Blakestown (Intel) 11 44 Whitehall Bull Tram (Luas) line Wadelai Park Larkhill Island Finglas Road Collins Avenue Principal stop Donnycarney St Anne’s Park 7b Bus route terminus Maynooth Ballymun and Gardens (DCU) Easton Glasnevin Cemetery Whitehall Marino Tram (Luas) line terminus Glasnevin Dublin (Mobhi) Harbour Maynooth St Patrick’s Fairview Transfer Points (Kingsbury) Prussia Street 66x Phibsboro Locations where it is possible to change Drumcondra North Strand to a different form of transport Leixlip Mountjoy Square Rail (DART, COMMUTER or Intercity) Salesian College 7b 7d 46e Mater Connolly/ 67x Phoenix Park Busáras (Infirmary Road Tram (Luas Red line) Phoenix Park and Zoo) 46a Parnell Square 116 Lucan Road Gardiner Bus coach (regional or intercity) (Liffey Valley) Palmerstown Street Backweston O’Connell Street Lucan Village Esker Hill Abbey Street Park & Ride (larger car parks) Lower Ballyoulster North Wall/Beckett Bridge Ferry Port Lucan Chapelizod (142 Outbound stop only) Dodsboro Bypass Dublin Port Aghards 25x Islandbridge Heuston Celbridge Points of Interest Grand Canal Dock 15a 15b 145 Public Park Heuston Arran/Usher’s -

Apartment 249 Fairbairn House, Bellevue, Islandbridge, Dublin 8 Apartment 249 Fairbairn House, Bellevue, Islandbridge, Dublin 8 for SALE by PRIVATE TREATY
Apartment 249 Fairbairn House, Bellevue, Islandbridge, Dublin 8 Apartment 249 Fairbairn House, Bellevue, Islandbridge, Dublin 8 FOR SALE BY PRIVATE TREATY Description Ganly Walters are delighted to present Apartment 249 Fairbairn House, Bellevue to the market. Bellevue is a highly regarded and much sought after modern development located on a stunning site on the banks of the River Liffey. Situated on the ground floor, this is a modern 2 bedroom apartment that is in walk in condition and extends to approximately 76 sq. m (818 sq. ft. approx.). This is an elegant and stylish apartment which has been refurbished and boasts light filled rooms and spacious accommodation. This is the perfect opportunity for those looking for a sound investment or a first time buyer. The accommodation includes entrance hallway, bathroom, two double bedrooms, spacious living/dining room with beautiful views over The River Liffey, modern fitted kitchen and utility room. Location Fairbairn House is well positioned central yet tranquil location and is within walking distance to Dublin City Centre and the Phoenix Park. Bellevue is a prime residential well maintained location with all amenities within a short stroll including, bus links, the Luas, Heuston Station. There is good access to both sides of the city centre, the M50 and many major national routes including the N4. Local attractions such as including Kilmainham Gaol, The War Memorial Gardens and The Irish Museum of Modern Art. Special Features • Large two bedroom apartment • Walk in condition • Superb city centre location • Easy access to the M50 and N4 • Wired for Alarm • Electric Storage Heating Directions From Heuston Station head west on Chapelizod Bypass/ St John’s Road, turn right onto South Circular Road. -

Liffey Estuary (Upper and Lower) Report 2010
ACKNOWLEDGEMENTS The authors wish to gratefully acknowledge the help and co -operation of the Director Mr. William Walsh and staff from IFI Blackrock as well as various other offices throughout the region. The authors also gratefully acknowledge the help and cooperation of their colleagues in IFI Swords . We would like to thank the landowners and angling clubs that granted us access to their land and respective fisheries. We would also like to thank Dr. Martin O’ Grady (IFI) and No. 3 Operational Wing, Irish Air Corps (Aer Chór na hÉireann) for the aerial photographs. PROJECT STAFF Project Director/Senior Research officer: Dr. Fiona Kelly Project Manager: Dr. Andrew Harrison Research Officer: Dr. Ronan Matson Research Officer: Ms. Lynda Connor Technician: Ms. Roisín O’Callaghan Technician Mr. Rory Feeney Technician: Ms. Emma Morrissey Technician: Mrs. Ciara Wögerbauer GIS Officer: Mr. Kieran Rocks Fisheries Assistant: Ms. Gráinne Hanna (Oct 2010 – Dec 2010) Fisheries Assistant: Mr. Kevin Gallagher (Oct 2010 – Dec 2010) The authors would also like to acknowledge the funding provided for the project from the Department of Communications Energy and Natural Resources for 2010. The report includes Ordnance Survey Ireland data reproduced under OS I Copyright Permit No. MP 007508. Unauthorised reproduction infringes Ordnance Survey Ireland and Government of Ireland copyright. © Ordnance Survey Ireland , 2010 TABLE OF CONTENTS 1. INTRODUCTION ................................ ................................ ................................ ............................. -

The Eucharistic Congress, 1932
Cultural and Environmental Education History THE EUCHARISTIC CONGRESS, 1932: helping students to assess historical significance November-December, 2012 Efforts have been made to trace and acknowledge copyright holders. In cases where a copyright has been inadvertently overlooked, the copyright holders are requested to contact the Cultural and Environmental Education Administrator, Catherine Begley, [email protected] © 2012 Cultural and Environmental Education, Professional Development Service for Teachers (PDST), 14 Joyce Way, Park West Business Park, Nangor Road, Dublin 12. 01-4358585, 01-4358596,[email protected], www.hist.ie © PDST, 2012 Page 1 Professional Development Service for Teachers (PDST) Cultural and Environmental Education History Contact details National Co-ordinator Conor Harrison Mobile 087 – 240 5710 E-mail [email protected] Administrator Catherine Begley Telephone 01-4358585 Fax 01-4358596 E-mail [email protected] Address 14 Joyce Way, ParkWestBusinessPark, Nangor Road, Dublin 12. Associate for History: John Dredge Acknowledgements With special thanks to Gerard O‟Sullivan, History Local Facilitator Thanks also to Dr. Rory O‟Dwyer, History Department, UCC. Note:Every effort has been made to ensure the accuracy of the historical data contained herein. Any inadvertent errors are regretted. © PDST, 2012 Page 2 CONTENTS Page The Eucharistic Congress, 1932: helping students to assess historical significance 4 The enquiry-focused approach 4 Considering the concept of historical significance 5 Proposed enquiry question: -

{PDF} Dublin : the City Within the Grand and Royal Canals and The
DUBLIN : THE CITY WITHIN THE GRAND AND ROYAL CANALS AND THE CIRCULAR ROAD, WITH THE PHOENIX PARK PDF, EPUB, EBOOK Christine Casey | 800 pages | 28 Feb 2006 | Yale University Press | 9780300109238 | English | New Haven, CT, United States Dublin : The City Within the Grand and Royal Canals and the Circular Road, with the Phoenix Park PDF Book Rich and varied house interiors are also treated in full, many for the first time. And have you any idea why it was called Micky Murry? We knew all the swans by their first names! The three elements that constitute the architectural legacy of Dublin—Norse, Norman , and Georgian —all meet in Dublin Castle. Very interesting; thanks, Dalgan. For anyone who is interested it is a very pleasant journey for the most part, the only difficult part being the section between Clonsilla and Coolmine where the canal is a good bit below the towpath and the path itself is quite narrow and strewn with the roots of trees. Is there a link I can use please? Notify me of new comments via email. We were enjoying it so much that we failed to notice the Vicar sneaking up behind us. Paul O'D Wednesday 25 January at Came across the site whilst browsing and thought it may be of interset to comment on the Spencer Dock lifting bridge to the canal. Freddie Hamilton Saturday 22 September at Language: English. It was completed in and is the seat of the archbishop of Dublin and primate of Ireland. How do series work? I have great memories from then. -

For Sale 18 Old Kilmainham, Kilmainham, Dublin 8
For Sale 18 Old Kilmainham, Kilmainham, Dublin 8 FILLER PICTURE RED OUTLINE FOR ILLUSTRATIVE PURPOSES ONLY Property Highlights Contact • Prime redevelopment opportunity in Kilmainham, a fashionable location in Dublin 8. Sam Carthy Email: [email protected] • Site extending to approx. 0.08 ha (0.19 acre) located on Old Tel: +353 1 639 9246 Kilmainham Road. John Donegan Email: [email protected] • Zoned Objective Z1 under the Dublin City Council Development Tel: +353 1 639 9222 Plan 2016-2022. Objective Z1 is defined as ‘To protect, provide and improve residential amenities’. Cushman & Wakefield • The area is mixed in nature with commercial, residential and 164 Shelbourne Road Ballsbridge, light industrial uses. Dublin 4 Ireland • The existing buildings extend to approx. 1,127 sq m (12,134 sq Tel: +353 (0)1 639 9300 ft). cushmanwakefield.ie Location The subject property is situated on the Old Kilmainham Road, close to the junction with Brookfield Road and approx. 250m east of the junction with South Circular Road. Kilmainham is a popular south west Dublin 8 suburb. The area is a popular and well established residential location with a mix of terraced residential housing and apartment blocks. There area a number of terraced residential properties along the main road with higher density apartments situated adjacent to the property and in the surrounding area. The property is situated close to the site of the new Children’s Hospital which has a planned opening date at the end of 2021. Public transport is provided by the Red Luas line with James Luas stop and Rialto Luas stop within 600m of the site.