The Cl to Ncl Branching Ratio in 248-Nm Photolysis of Chlorine Azide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potentially Explosive Chemicals*
Potentially Explosive Chemicals* Chemical Name CAS # Not 1,1’-Diazoaminonaphthalene Assigned 1,1-Dinitroethane 000600-40-8 1,2,4-Butanetriol trinitrate 006659-60-5 1,2-Diazidoethane 000629-13-0 1,3,5-trimethyl-2,4,6-trinitrobenzene 000602-96-0 1,3-Diazopropane 005239-06-5 Not 1,3-Dinitro-4,5-dinitrosobenzene Assigned Not 1,3-dinitro-5,5-dimethyl hydantoin Assigned Not 1,4-Dinitro-1,1,4,4-tetramethylolbutanetetranitrate Assigned Not 1,7-Octadiene-3,5-Diyne-1,8-Dimethoxy-9-Octadecynoic acid Assigned 1,8 –dihydroxy 2,4,5,7-tetranitroanthraquinone 000517-92-0 Not 1,9-Dinitroxy pentamethylene-2,4,6,8-tetramine Assigned 1-Bromo-3-nitrobenzene 000585-79-5 Not 2,2',4,4',6,6'-Hexanitro-3,3'-dihydroxyazobenzene Assigned 2,2-di-(4,4,-di-tert-butylperoxycyclohexyl)propane 001705-60-8 2,2-Dinitrostilbene 006275-02-1 2,3,4,6- tetranitrophenol 000641-16-7 Not 2,3,4,6-tetranitrophenyl methyl nitramine Assigned Not 2,3,4,6-tetranitrophenyl nitramine Assigned Not 2,3,5,6- tetranitroso nitrobenzene Assigned Not 2,3,5,6- tetranitroso-1,4-dinitrobenzene Assigned 2,4,6-Trinitro-1,3,5-triazo benzene 029306-57-8 Not 2,4,6-trinitro-1,3-diazabenzene Assigned Not 2,4,6-Trinitrophenyl trimethylol methyl nitramine trinitrate Assigned Not 2,4,6-Trinitroso-3-methyl nitraminoanisole Assigned 2,4-Dinitro-1,3,5-trimethyl-benzene 000608-50-4 2,4-Dinitrophenylhydrazine 000119-26-6 2,4-Dinitroresorcinol 000519-44-8 2,5-dimethyl-2,5-diydroperoxy hexane 2-Nitro-2-methylpropanol nitrate 024884-69-3 3,5-Dinitrosalicylic acid 000609-99-4 Not 3-Azido-1,2-propylene glycol dinitrate -

List of Reactive Chemicals
LIST OF REACTIVE CHEMICALS Chemical Prefix Chemical Name Reactive Reactive Reactive CAS# Chemical Chemical Chemical Stimulus 1 Stimulus 2 Stimulus 3 111-90-0 "CARBITOL" SOLVENT D 111-15-9 "CELLOSOLVE" ACETATE D 110-80-5 "CELLOSOLVE" SOLVENT D 2- (2,4,6-TRINITROPHENYL)ETHYL ACETATE (1% IN ACETONE & BENZENE S 12427-38-2 AAMANGAN W 88-85-7 AATOX S 40487-42-1 AC 92553 S 105-57-7 ACETAL D 75-07-0 ACETALDEHYDE D 105-57-7 ACETALDEHYDE, DIETHYL ACETAL D 108-05-4 ACETIC ACID ETHENYL ESTER D 108-05-4 ACETIC ACID VINYL ESTER D 75-07-0 ACETIC ALDEHYDE D 101-25-7 ACETO DNPT T 126-84-1 ACETONE DIETHYL ACETAL D 108-05-4 ACETOXYETHYLENE D 108-05-4 1- ACETOXYETHYLENE D 37187-22-7 ACETYL ACETONE PEROXIDE, <=32% AS A PASTE T 37187-22-7 ACETYL ACETONE PEROXIDE, <=42% T 37187-22-7 ACETYL ACETONE PEROXIDE, >42% T S 644-31-5 ACETYL BENZOYL PEROXIDE (SOLID OR MORE THAN 45% IN SOLUTION) T S 644-31-5 ACETYL BENZOYL PEROXIDE, <=45% T 506-96-7 ACETYL BROMIDE W 75-36-5 ACETYL CHLORIDE W ACETYL CYCLOHEXANE SULFONYL PEROXIDE (>82% WITH <12% WATER) T S 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=32% T 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=82% T 674-82-8 ACETYL KETENE (POISON INHALATION HAZARD) D 110-22-5 ACETYL PEROXIDE, <=27% T 110-22-5 ACETYL PEROXIDE, SOLID, OR MORE THAN 27% IN SOLUTION T S 927-86-6 ACETYLCHOLINE PERCHLORATE O S 74-86-2 ACETYLENE D 74-86-2 ACETYLENE (LIQUID) D ACETYLENE SILVER NITRATE D 107-02-08 ACRALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACROLEIC ACID D 107-02-08 ACROLEIN, INHIBITED (POISON INHALATION HAZARD) D 107-02-08 ACRYLALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACRYLIC ACID D 141-32-2 ACRYLIC ACID BUTYL ESTER D 140-88-5 ACRYLIC ACID ETHYL ESTER D 96-33-3 ACRYLIC ACID METHYL ESTER D Stimulus - Stimuli is the thermal, physical or chemical input needed to induce a hazardous reaction. -

Hazardous Materials Table Quirements
Pipeline and Hazardous Materials Safety Admin., DOT § 172.101 Subpart H—Training this part, that person shall perform the function in accordance with this part. 172.700 Purpose and scope. 172.701 Federal-State relationship. [Amdt. 172–29, 41 FR 15996, Apr. 15, 1976, as 172.702 Applicability and responsibility for amended by Amdt. 172–32, 41 FR 38179, Sept. training and testing. 9, 1976] 172.704 Training requirements. Subpart B—Table of Hazardous Subpart I—Safety and Security Plans Materials and Special Provisions 172.800 Purpose and applicability. § 172.101 Purpose and use of haz- 172.802 Components of a security plan. ardous materials table. 172.804 Relationship to other Federal re- (a) The Hazardous Materials Table quirements. (Table) in this section designates the 172.820 Additional planning requirements for transportation by rail. materials listed therein as hazardous 172.822 Limitation on actions by states, materials for the purpose of transpor- local governments, and Indian tribes. tation of those materials. For each listed material, the Table identifies the APPENDIX A TO PART 172—OFFICE OF HAZ- hazard class or specifies that the mate- ARDOUS MATERIALS TRANSPORTATION rial is forbidden in transportation, and COLOR TOLERANCE CHARTS AND TABLES gives the proper shipping name or di- APPENDIX B TO PART 172—TREFOIL SYMBOL rects the user to the preferred proper APPENDIX C TO PART 172—DIMENSIONAL SPEC- IFICATIONS FOR RECOMMENDED PLACARD shipping name. In addition, the Table HOLDER specifies or references requirements in APPENDIX D TO PART 172—RAIL RISK ANAL- this subchapter pertaining to labeling, YSIS FACTORS packaging, quantity limits aboard air- craft and stowage of hazardous mate- AUTHORITY: 49 U.S.C. -

CHEM Safety Manual
Department of Chemistry Safety Manual October 2018 Safety Committee Department of Chemistry Hong Kong University of Science and Technology Table of Contents 1.0 Introduction 2.0 Safety Policy and Responsibility for Safety 2.1 Department Head 2.2 Department of Chemistry Safety Committee 2.3 Laboratory Supervisors 2.4 Researchers 2.5 HSEO 3.0 Information, Training, Safety Clearance, and Safety Clearance at Termination 3.1 Initial Training 3.2 Information on Hazardous Substances 3.3 Additional Safety Information 3.4 Safety Clearance at Termination 4.0 Personal Protective Equipment and Safety Engineering Controls 4.1 Eye Protection 4.2 Protective Apparel 4.3 Respirators 4.4 Laboratory Fume Cupboards 4.5 Fire Extinguishers, Safety Showers, and Eyewash Facilities 5.0 Standard Operating Procedures for Work with Hazardous Substances 5.1 Classes of Hazardous Substances 5.2 General Procedures for Work with Toxic Substances 5.3 General Procedures for Work with Flammable and Explosive Substances 6.0 Procedures for Work with Particularly Hazardous Substances 6.1 Identification and Classification of Particularly Hazardous Substances 6.2 Designated Areas 6.3 General Procedures for Work with Substances of Moderate to High Chronic or High Acute Toxicity 6.4 Additional Procedures for Work with Substances of Known High Chronic Toxicity 6.5 Specific Handling Procedures for Some Common Particularly Hazardous Substances 7.0 Proper Planning of Laboratory Work 7.1 Recognition and Assessment 7.2 Planning for the Unexpected: What Could Go Wrong? 7.3 Site Selection -

Polymeric Nitrogen by Plasma Enhanced Chemical Vapor Deposition
New Jersey Institute of Technology Digital Commons @ NJIT Dissertations Electronic Theses and Dissertations Fall 1-31-2015 Polymeric nitrogen by plasma enhanced chemical vapor deposition El Mostafa Benchafia New Jersey Institute of Technology Follow this and additional works at: https://digitalcommons.njit.edu/dissertations Part of the Materials Science and Engineering Commons Recommended Citation Benchafia, El Mostafa, "Polymeric nitrogen by plasma enhanced chemical vapor deposition" (2015). Dissertations. 98. https://digitalcommons.njit.edu/dissertations/98 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at Digital Commons @ NJIT. It has been accepted for inclusion in Dissertations by an authorized administrator of Digital Commons @ NJIT. For more information, please contact [email protected]. Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept -
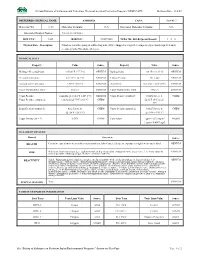
Nevada Division of Environmental Protection, Chemical Accident Prevention Program (NDEP-CAPP) Revision Date: 11/2/07
Nevada Division of Environmental Protection, Chemical Accident Prevention Program (NDEP-CAPP) Revision Date: 11/2/07 PREFERRED CHEMICAL NAME: AMMONIA CAS #: 7664-41-7 Molecular Wt. 17.03 Molecular Formula: H3N Structural Molecular Formula: NH3 Alternate Chemical Names: Anhydrous Ammonia DOT UN # : 1005 RTECS # : BO0875000 NFPA 704: H-F-R-Special Hazard: 3 – 0 – 0 Physical State - Description: Colorless gas with a pungent, suffocating odor. [Note: Shipped as a liquefied compressed gas. Easily liquefied under pressure.]; Odor Threshold: 46.8 ppm PHYSICAL DATA Property Value Source Property Value Source Melting – Freezing Point: -107.86°F (-77.7°C) GENIUM Boiling Point: -28 °F (-33.35°C) GENIUM Critical Temperature: 270.3°F (132.4°C) GENIUM Critical Pressure: 111.5 atm GENIUM Autoignition Temperature: 1204°F (651°C) GENIUM Flash Point: Indefinite <32°F (0°C) GENIUM Lower Flammability Limit: 16% v/v GENIUM Upper Flammability Limit: 25% v/v GENIUM Vapor Pressure: 1 mm Hg @ -164.4°F (-109.1°C) GENIUM Vapor Density (standard): 0.0482 lbs/cu. ft. CHEM Vapor Pressure (saturated): 128.8 psia @ 70°F (21.1°C) CHEM @ 32°F (0°C) and 1 atm Liquid Density (saturated): 42.2 lbs/cu. ft. CHEM Vapor Density (saturated): 0.0527 lbs/cu. ft. CHEM @ -20°F (-28.9°C) @ -30°F (-34.4°C) Vapor Density (air = 1): 0.5971 CHEM Conversion: 1 ppm = 0.70 mg/m3 NIOSH 1 ppm = 0.0007 mg/l HAZARD OVERVIEWS Hazard Overview Source HEALTH Corrosive causes burns to eyes/skin/respiratory tract. Also Causes: blindness; exposure to high levels may be fatal. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 11/14/2019 Revision date 11/14/2019 Revision Number: G1 1. IDENTIFICATION Product identifier Product code: A-323 Product Name: AMMONIUM CHLORIDE-AMMONIUM HYDROXIDE TS, (U.S.P. TEST SOLUTION) Other means of identification Synonyms: No information available CAS #: Mixture RTECS # Not available CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Standardized solution. Laboratory reagent. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Tom Tyner (USA - West Coast) Contact Person: Ibad Tirmiz (USA - East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Inhalation (Vapors) Category 4 Skin corrosion/irritation Category 1 Serious eye damage/eye irritation Category 1 Specific target organ toxicity (single exposure) Category 3 Label elements Danger Hazard statements Harmful if inhaled Causes severe skin burns and eye damage May cause respiratory irritation Product code: A-323 Product name: AMMONIUM Page 1 / 16 CHLORIDE-AMMONIUM HYDROXIDE TS, (U.S.P. TEST SOLUTION) Hazards not otherwise classified (HNOC) Not Applicable Other hazards May be harmful if swallowed Precautionary Statements - Prevention Use only outdoors or in a well-ventilated area Do not breathe mist or vapors Wash face, hands and any exposed skin thoroughly after handling Wear protective gloves/protective clothing/eye protection/face protection Precautionary Statements - Response Immediately call a POISON CENTER or physician IF IN EYES: Rinse cautiously with water for several minutes. -

Velocity Map Ion Imaging of Chlorine Azide Photolysis: Evidence for Photolytic † Production of Cyclic-N3
Velocity Map Ion Imaging of Chlorine Azide Photolysis: Evidence for Photolytic † Production of Cyclic-N3 N. Hansen and A. M. Wodtke* Department of Chemistry and Biochemistry, UniVersity of California at Santa Barbara, Santa Barbara, California 93106 ReceiVed: March 13, 2003 The method of velocity map imaging was applied to study the photodissociation dynamics of ClN3 near 235 2 nm under collision-free conditions. Derived kinetic energy distributions of state-selected Cl ( PJ) provide a medium-resolution energy spectrum of the N3 fragment. Markedly bimodal distributions are observed that 2 suggest simultaneous formation of the linear-N3 (X˜ Π) isomer as well as an energetic form of N3, consistent with recent theoretical predictions of a cyclic isomer, resembling an isosceles triangle. Angular distributions of the photofragments indicate that 21A′ r 11A′ excitation is the most important pathway to photoproducts. 2 Branching ratio measurements between the dominant spin-orbit excited-state Cl* ( P1/2) and the spin-orbit 2 ground-state Cl ( P3/2) showed Cl*/Cl ≈ 0.8/0.2. The branching ratio between linear-N3 and cyclic-N3 formation was determined to be ≈ 0.8/0.2. Introduction ˜ 1 ′ + f 3 + 3 + ClN3 (X Α ) hν NCl (X Σ) N2 (A Σg ) (4) Recent interest in the photochemistry of chlorine azide (ClN3) 3 ∑+ 5,6 derives from demonstrations that the primary photoproduct NCl N2 (A g ), presumably from channel 4, has been detected, (a 1∆) can be used as an effective energy carrier in chemical along with NCl (b 1 Σ+) which can be produced by a second 1-3 5,13 iodine lasers. -
Properties and Hazards of 108 Selected Substances -1992 Edition
U. S. DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY PROPERTIES AND HAZARDS OF 108 SELECTED SUBSTANCES -1992 EDITION Jeffrey E. Lucius1, Gary R. Olhoeft1, Patricia L. Hill1, and Steven K. Duke2 U.S. Geological Survey Open-File Report 92-527 September 1992 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. 1 Golden, CO, USA 2 now with Woodward-Clyde Consultants, 2020 E. First Street, Suite 900, Santa Ana, CA 92705 iii CONTENTS List of Tables ....................................................... vi Notice ............................................................... vii Introduction ......................................................... 1 Properties Definitions ................................................... 4 Key to Abbreviations .......................................... 13 Conversion Factors ............................................ 16 Tables .............................................................. 18 Substances .............................. GAS RN Acetic acid .................... 64-19-7 .................. 70 Acetone ........................ 67-64-1 .................. 75 Acrolein ....................... 107-02-8 .................. 81 Acrylonitrile .................. 107-13-1 .................. 85 Aldrin ......................... 309-00-2 .................. 89 Ammonia ........................ 7664-41-7 .................. 92 -

Common Name: DIETHYLALUMINUM CHLORIDE HAZARD SUMMARY
Common Name: DIETHYLALUMINUM CHLORIDE CAS Number: 96-10-6 RTK Substance number: 0689 DOT Number: UN 3052 Date: December 1995 Revision: June 2001 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Diethylaluminum Chloride can affect you when breathed * If you think you are experiencing any work-related health in. problems, see a doctor trained to recognize occupational * Contact can severely irritate and burn the skin and eyes diseases. Take this Fact Sheet with you. with possible eye damage. * Breathing Diethylaluminum Chloride can irritate the WORKPLACE EXPOSURE LIMITS nose, throat and lungs causing coughing, wheezing and/or The following exposure limits are for Aluminum Alkyls shortness of breath. (measured as Aluminum): * Diethylaluminum Chloride is a HIGHLY FLAMMABLE and REACTIVE chemical and a NIOSH: The recommended airborne exposure limit is DANGEROUS FIRE and EXPLOSION HAZARD. 2 mg/m3 averaged over a 10-hour workshift. IDENTIFICATION ACGIH: The recommended airborne exposure limit is Diethylaluminum Chloride is a colorless liquid which 2 mg/m3 averaged over an 8-hour workshift. ignites instantly on contact with air. It is often shipped in solution with a Hydrocarbon solvent. It is used as a catalyst WAYS OF REDUCING EXPOSURE and as an intermediate in the production of other chemicals. * Where possible, enclose operations and use local exhaust ventilation at the site of chemical release. If local exhaust REASON FOR CITATION ventilation or enclosure is not used, respirators should be * Diethylaluminum Chloride is on the Hazardous worn. Substance List because it is cited by ACGIH, DOT, * Wear protective work clothing. NIOSH and NFPA. * Wash thoroughly immediately after exposure to * This chemical is on the Special Health Hazard Substance Diethylaluminum Chloride and at the end of the List because it is FLAMMABLE and REACTIVE. -

Anhydrous Ammonia
Anhydrous Ammonia Safety Data Sheet (SDS) USS IHS Number: 44 (Replaces USS Code Number: 5B81-83) Location(s): Clairton Original: 12/16/2010 Revision: 6/21/2017 Expiration: 6/21/2020 Section 1 – Identification 1(a) Product Identifier Used on Label: Anhydrous Ammonia 1(b) Other Means of Identification: Ammonia 1(c) Recommended Use of the Chemical and Restrictions on Use: None 1(d) Name, Address, and Telephone Number: United States Steel Corporation Phone number: (412) 433-6840 (8:00 am to 5:00 pm) 600 Grant Street, Room 1662 FAX: (412) 433-5019 Pittsburgh, PA 15219-2800 1(e) Emergency Phone Number: 1-800-262-8200 (CHEMTREC) Section 2 – Hazard(s) Identification 2(a) Classification of the Chemical: Anhydrous Ammonia is considered a hazardous material according to the criteria specified in REACH [REGULATION (EC) No 1907/2006] and CLP [REGULATION (EC) No 1272/2008] and OSHA 29 CFR 1910.1200 Hazard Communication Standard. The categories of Health Hazards as defined in “GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELLING OF CHEMICALS (GHS), Third revised edition ST/SG/AC.10/30/Rev. 3” United Nations, New York and Geneva, 2009 have been evaluated. Refer to Section 3, 8 and 11 for additional information. 2(b) Signal Word, Hazard Statement(s), Symbols and Precautionary Statement(s): Hazard Hazard Classification Signal Word Hazard Statement(s) Precautionary Statement(s) Symbol Do not breathe gas/mist/vapors or spray. Acute Toxicity Use only outdoors or in a well-ventilated area. Inhalation - 3 Toxic if inhaled. Wear protective gloves / protective clothing / eye protection / face Danger Causes severe skin burns protection. -

Organic & Biomolecular Chemistry
Organic & Biomolecular Chemistry Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/obc Page 1 of 5 OrganicPlease & doBiomolecular not adjust margins Chemistry Journal Name COMMUNICATION Safe generation and use of bromine azide under continuous flow conditions ̶ selective 1,2-bromoazidation of olefins Received 00th January 20xx, Accepted 00th January 20xx David Cantillo, Bernhard Gutmann and C. Oliver Kappe* Manuscript DOI: 10.1039/x0xx00000x www.rsc.org/ Bromine azide (BrN3), a useful but extremely toxic and explosive halogen azides was systematically explored by the group of reagent for the preparation of vicinal 1,2-bromine azide Hassner.4 Hassner and co-workers for the first time compounds, was safely generated and reacted in situ with alkenes demonstrated the direct addition of halogen azides to olefins in a continuous flow photoreactor.