Material Safety Data Sheet Ammonia-Ammonium Chloride Buffer TS MSDS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Durham E-Theses
Durham E-Theses A spectroscopic study of some halogeno-complexes of tellurium (IV) Gorrell, Ian Barnes How to cite: Gorrell, Ian Barnes (1983) A spectroscopic study of some halogeno-complexes of tellurium (IV), Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/7890/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk A SPECTROSCOPIC STUDY OF SOME HALOGENO- COMPLEXES OF TELLURIUM (IV) by Ian Barnes Gorrell A thesis submitted in part fulfilment of the requirements for the degree of Master of Science in the University of Durham. The copyright of this thesis rests with the author. April 19 8 3 No quotation from it should be published without his prior written consent and information derived from it should be acknowledged. -5. OFC. 1983 TO MY MOTHER and MY FATHER1 S MEMORY' iii ACKNOWLEDGMENTS I wish to express my gratitude towards the late Professor T.C. -

Potentially Explosive Chemicals*
Potentially Explosive Chemicals* Chemical Name CAS # Not 1,1’-Diazoaminonaphthalene Assigned 1,1-Dinitroethane 000600-40-8 1,2,4-Butanetriol trinitrate 006659-60-5 1,2-Diazidoethane 000629-13-0 1,3,5-trimethyl-2,4,6-trinitrobenzene 000602-96-0 1,3-Diazopropane 005239-06-5 Not 1,3-Dinitro-4,5-dinitrosobenzene Assigned Not 1,3-dinitro-5,5-dimethyl hydantoin Assigned Not 1,4-Dinitro-1,1,4,4-tetramethylolbutanetetranitrate Assigned Not 1,7-Octadiene-3,5-Diyne-1,8-Dimethoxy-9-Octadecynoic acid Assigned 1,8 –dihydroxy 2,4,5,7-tetranitroanthraquinone 000517-92-0 Not 1,9-Dinitroxy pentamethylene-2,4,6,8-tetramine Assigned 1-Bromo-3-nitrobenzene 000585-79-5 Not 2,2',4,4',6,6'-Hexanitro-3,3'-dihydroxyazobenzene Assigned 2,2-di-(4,4,-di-tert-butylperoxycyclohexyl)propane 001705-60-8 2,2-Dinitrostilbene 006275-02-1 2,3,4,6- tetranitrophenol 000641-16-7 Not 2,3,4,6-tetranitrophenyl methyl nitramine Assigned Not 2,3,4,6-tetranitrophenyl nitramine Assigned Not 2,3,5,6- tetranitroso nitrobenzene Assigned Not 2,3,5,6- tetranitroso-1,4-dinitrobenzene Assigned 2,4,6-Trinitro-1,3,5-triazo benzene 029306-57-8 Not 2,4,6-trinitro-1,3-diazabenzene Assigned Not 2,4,6-Trinitrophenyl trimethylol methyl nitramine trinitrate Assigned Not 2,4,6-Trinitroso-3-methyl nitraminoanisole Assigned 2,4-Dinitro-1,3,5-trimethyl-benzene 000608-50-4 2,4-Dinitrophenylhydrazine 000119-26-6 2,4-Dinitroresorcinol 000519-44-8 2,5-dimethyl-2,5-diydroperoxy hexane 2-Nitro-2-methylpropanol nitrate 024884-69-3 3,5-Dinitrosalicylic acid 000609-99-4 Not 3-Azido-1,2-propylene glycol dinitrate -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

List of Reactive Chemicals
LIST OF REACTIVE CHEMICALS Chemical Prefix Chemical Name Reactive Reactive Reactive CAS# Chemical Chemical Chemical Stimulus 1 Stimulus 2 Stimulus 3 111-90-0 "CARBITOL" SOLVENT D 111-15-9 "CELLOSOLVE" ACETATE D 110-80-5 "CELLOSOLVE" SOLVENT D 2- (2,4,6-TRINITROPHENYL)ETHYL ACETATE (1% IN ACETONE & BENZENE S 12427-38-2 AAMANGAN W 88-85-7 AATOX S 40487-42-1 AC 92553 S 105-57-7 ACETAL D 75-07-0 ACETALDEHYDE D 105-57-7 ACETALDEHYDE, DIETHYL ACETAL D 108-05-4 ACETIC ACID ETHENYL ESTER D 108-05-4 ACETIC ACID VINYL ESTER D 75-07-0 ACETIC ALDEHYDE D 101-25-7 ACETO DNPT T 126-84-1 ACETONE DIETHYL ACETAL D 108-05-4 ACETOXYETHYLENE D 108-05-4 1- ACETOXYETHYLENE D 37187-22-7 ACETYL ACETONE PEROXIDE, <=32% AS A PASTE T 37187-22-7 ACETYL ACETONE PEROXIDE, <=42% T 37187-22-7 ACETYL ACETONE PEROXIDE, >42% T S 644-31-5 ACETYL BENZOYL PEROXIDE (SOLID OR MORE THAN 45% IN SOLUTION) T S 644-31-5 ACETYL BENZOYL PEROXIDE, <=45% T 506-96-7 ACETYL BROMIDE W 75-36-5 ACETYL CHLORIDE W ACETYL CYCLOHEXANE SULFONYL PEROXIDE (>82% WITH <12% WATER) T S 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=32% T 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=82% T 674-82-8 ACETYL KETENE (POISON INHALATION HAZARD) D 110-22-5 ACETYL PEROXIDE, <=27% T 110-22-5 ACETYL PEROXIDE, SOLID, OR MORE THAN 27% IN SOLUTION T S 927-86-6 ACETYLCHOLINE PERCHLORATE O S 74-86-2 ACETYLENE D 74-86-2 ACETYLENE (LIQUID) D ACETYLENE SILVER NITRATE D 107-02-08 ACRALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACROLEIC ACID D 107-02-08 ACROLEIN, INHIBITED (POISON INHALATION HAZARD) D 107-02-08 ACRYLALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACRYLIC ACID D 141-32-2 ACRYLIC ACID BUTYL ESTER D 140-88-5 ACRYLIC ACID ETHYL ESTER D 96-33-3 ACRYLIC ACID METHYL ESTER D Stimulus - Stimuli is the thermal, physical or chemical input needed to induce a hazardous reaction. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Hazardous Materials Table Quirements
Pipeline and Hazardous Materials Safety Admin., DOT § 172.101 Subpart H—Training this part, that person shall perform the function in accordance with this part. 172.700 Purpose and scope. 172.701 Federal-State relationship. [Amdt. 172–29, 41 FR 15996, Apr. 15, 1976, as 172.702 Applicability and responsibility for amended by Amdt. 172–32, 41 FR 38179, Sept. training and testing. 9, 1976] 172.704 Training requirements. Subpart B—Table of Hazardous Subpart I—Safety and Security Plans Materials and Special Provisions 172.800 Purpose and applicability. § 172.101 Purpose and use of haz- 172.802 Components of a security plan. ardous materials table. 172.804 Relationship to other Federal re- (a) The Hazardous Materials Table quirements. (Table) in this section designates the 172.820 Additional planning requirements for transportation by rail. materials listed therein as hazardous 172.822 Limitation on actions by states, materials for the purpose of transpor- local governments, and Indian tribes. tation of those materials. For each listed material, the Table identifies the APPENDIX A TO PART 172—OFFICE OF HAZ- hazard class or specifies that the mate- ARDOUS MATERIALS TRANSPORTATION rial is forbidden in transportation, and COLOR TOLERANCE CHARTS AND TABLES gives the proper shipping name or di- APPENDIX B TO PART 172—TREFOIL SYMBOL rects the user to the preferred proper APPENDIX C TO PART 172—DIMENSIONAL SPEC- IFICATIONS FOR RECOMMENDED PLACARD shipping name. In addition, the Table HOLDER specifies or references requirements in APPENDIX D TO PART 172—RAIL RISK ANAL- this subchapter pertaining to labeling, YSIS FACTORS packaging, quantity limits aboard air- craft and stowage of hazardous mate- AUTHORITY: 49 U.S.C. -

CHEM Safety Manual
Department of Chemistry Safety Manual October 2018 Safety Committee Department of Chemistry Hong Kong University of Science and Technology Table of Contents 1.0 Introduction 2.0 Safety Policy and Responsibility for Safety 2.1 Department Head 2.2 Department of Chemistry Safety Committee 2.3 Laboratory Supervisors 2.4 Researchers 2.5 HSEO 3.0 Information, Training, Safety Clearance, and Safety Clearance at Termination 3.1 Initial Training 3.2 Information on Hazardous Substances 3.3 Additional Safety Information 3.4 Safety Clearance at Termination 4.0 Personal Protective Equipment and Safety Engineering Controls 4.1 Eye Protection 4.2 Protective Apparel 4.3 Respirators 4.4 Laboratory Fume Cupboards 4.5 Fire Extinguishers, Safety Showers, and Eyewash Facilities 5.0 Standard Operating Procedures for Work with Hazardous Substances 5.1 Classes of Hazardous Substances 5.2 General Procedures for Work with Toxic Substances 5.3 General Procedures for Work with Flammable and Explosive Substances 6.0 Procedures for Work with Particularly Hazardous Substances 6.1 Identification and Classification of Particularly Hazardous Substances 6.2 Designated Areas 6.3 General Procedures for Work with Substances of Moderate to High Chronic or High Acute Toxicity 6.4 Additional Procedures for Work with Substances of Known High Chronic Toxicity 6.5 Specific Handling Procedures for Some Common Particularly Hazardous Substances 7.0 Proper Planning of Laboratory Work 7.1 Recognition and Assessment 7.2 Planning for the Unexpected: What Could Go Wrong? 7.3 Site Selection -

Polymeric Nitrogen by Plasma Enhanced Chemical Vapor Deposition
New Jersey Institute of Technology Digital Commons @ NJIT Dissertations Electronic Theses and Dissertations Fall 1-31-2015 Polymeric nitrogen by plasma enhanced chemical vapor deposition El Mostafa Benchafia New Jersey Institute of Technology Follow this and additional works at: https://digitalcommons.njit.edu/dissertations Part of the Materials Science and Engineering Commons Recommended Citation Benchafia, El Mostafa, "Polymeric nitrogen by plasma enhanced chemical vapor deposition" (2015). Dissertations. 98. https://digitalcommons.njit.edu/dissertations/98 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at Digital Commons @ NJIT. It has been accepted for inclusion in Dissertations by an authorized administrator of Digital Commons @ NJIT. For more information, please contact [email protected]. Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 12/22/2016 Revision Date: 12/22/2016 Revision Number: G1 1. IDENTIFICATION Product identifier Product code: A-540 Product Name: AMMONIUM HYDROXIDE, 6.0 N SOLUTION Other means of identification Synonyms: No information available CAS #: Mixture RTECS # Not available CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: No information available. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000. Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous according to the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Skin corrosion/irritation Category 1 Sub-category B Serious eye damage/eye irritation Category 1 Specific target organ toxicity (single exposure) Category 3 Label elements Danger Hazard statements Causes severe skin burns and eye damage May cause respiratory irritation Product code: A-540 Product name: AMMONIUM 1 / 13 HYDROXIDE, 6.0 N SOLUTION Hazards not otherwise classified (HNOC) Not Applicable Other hazards May be harmful if swallowed May be harmful if inhaled Precautionary Statements - Prevention Do not breathe dust/fume/gas/mist/vapors/spray Wash face, hands and any exposed skin thoroughly after handling Wear protective gloves/protective clothing/eye protection/face protection Use only outdoors or in a well-ventilated area Precautionary Statements - Response Immediately call a POISON CENTER or doctor/physician IF IN EYES: Rinse cautiously with water for several minutes. -
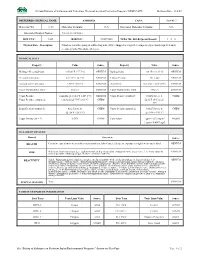
Nevada Division of Environmental Protection, Chemical Accident Prevention Program (NDEP-CAPP) Revision Date: 11/2/07
Nevada Division of Environmental Protection, Chemical Accident Prevention Program (NDEP-CAPP) Revision Date: 11/2/07 PREFERRED CHEMICAL NAME: AMMONIA CAS #: 7664-41-7 Molecular Wt. 17.03 Molecular Formula: H3N Structural Molecular Formula: NH3 Alternate Chemical Names: Anhydrous Ammonia DOT UN # : 1005 RTECS # : BO0875000 NFPA 704: H-F-R-Special Hazard: 3 – 0 – 0 Physical State - Description: Colorless gas with a pungent, suffocating odor. [Note: Shipped as a liquefied compressed gas. Easily liquefied under pressure.]; Odor Threshold: 46.8 ppm PHYSICAL DATA Property Value Source Property Value Source Melting – Freezing Point: -107.86°F (-77.7°C) GENIUM Boiling Point: -28 °F (-33.35°C) GENIUM Critical Temperature: 270.3°F (132.4°C) GENIUM Critical Pressure: 111.5 atm GENIUM Autoignition Temperature: 1204°F (651°C) GENIUM Flash Point: Indefinite <32°F (0°C) GENIUM Lower Flammability Limit: 16% v/v GENIUM Upper Flammability Limit: 25% v/v GENIUM Vapor Pressure: 1 mm Hg @ -164.4°F (-109.1°C) GENIUM Vapor Density (standard): 0.0482 lbs/cu. ft. CHEM Vapor Pressure (saturated): 128.8 psia @ 70°F (21.1°C) CHEM @ 32°F (0°C) and 1 atm Liquid Density (saturated): 42.2 lbs/cu. ft. CHEM Vapor Density (saturated): 0.0527 lbs/cu. ft. CHEM @ -20°F (-28.9°C) @ -30°F (-34.4°C) Vapor Density (air = 1): 0.5971 CHEM Conversion: 1 ppm = 0.70 mg/m3 NIOSH 1 ppm = 0.0007 mg/l HAZARD OVERVIEWS Hazard Overview Source HEALTH Corrosive causes burns to eyes/skin/respiratory tract. Also Causes: blindness; exposure to high levels may be fatal. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 11/14/2019 Revision date 11/14/2019 Revision Number: G1 1. IDENTIFICATION Product identifier Product code: A-323 Product Name: AMMONIUM CHLORIDE-AMMONIUM HYDROXIDE TS, (U.S.P. TEST SOLUTION) Other means of identification Synonyms: No information available CAS #: Mixture RTECS # Not available CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Standardized solution. Laboratory reagent. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Tom Tyner (USA - West Coast) Contact Person: Ibad Tirmiz (USA - East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Inhalation (Vapors) Category 4 Skin corrosion/irritation Category 1 Serious eye damage/eye irritation Category 1 Specific target organ toxicity (single exposure) Category 3 Label elements Danger Hazard statements Harmful if inhaled Causes severe skin burns and eye damage May cause respiratory irritation Product code: A-323 Product name: AMMONIUM Page 1 / 16 CHLORIDE-AMMONIUM HYDROXIDE TS, (U.S.P. TEST SOLUTION) Hazards not otherwise classified (HNOC) Not Applicable Other hazards May be harmful if swallowed Precautionary Statements - Prevention Use only outdoors or in a well-ventilated area Do not breathe mist or vapors Wash face, hands and any exposed skin thoroughly after handling Wear protective gloves/protective clothing/eye protection/face protection Precautionary Statements - Response Immediately call a POISON CENTER or physician IF IN EYES: Rinse cautiously with water for several minutes.