Optical Constants of Evaporated Amorphous Zinc Arsenide (Zn 3 As 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
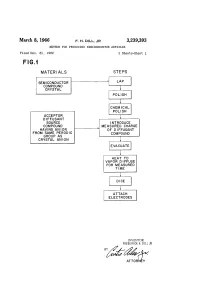
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD for PRODUCING SEMICONDUCTOR ARTICLES Filed Dec
March 8, 1966 F. H. DIL, JR 3,239,393 METHOD FOR PRODUCING SEMICONDUCTOR ARTICLES Filed Dec. 31, 1962 2. Sheets-Sheet FG. MATER ALS STEPS SEMCONDUCTOR COMPOUND CRYSTAL POLISH CHEMICAL POLISH ACCEPTOR DFFUSANT SOURCE INTRODUCE COMPOUND MEASURED CHARGE HAVING AN ON OF DFFUSANT FROM SAME PERODC COMPOUND GROUP AS CRYSTAL ANION EVACUATE HEAT TO VAPOR DIFFUSE FOR MEASURED TIME ATTACH ELECTRODES INVENTOR. FREDERICK H. DLL JR ATTORNEY March 8, 1966 F., H., DL, JR 3,239,393 METHOD FOR PRODUCING SEMCONDUCTOR ARTICLES Filed Dec. 31, 962 2. Sheets-Sheet 2 Šes is series NNNNN r N NNNNNNNN 3,239,393 United States Patent Office Patented Mar. 8, 1966 2. to produce precisely the desired results. For instance, 3,239,393 when metallic zinc is used as the diffusant material for METHOD FOR PRODUCING SEMCONDUCTOR a gallium arsenide substrate, it is very difficult to obtain ARTICLES the pure zinc metal with no zinc oxide film upon the metal. Frederick H. Dii, Jr., Patnam Waley, N.Y., assigor to 5 Furthermore, the metal is so tough that it is difficult to International Business Machines Corporation, New divide a pure metal sample into smaller pieces in order York, N.Y., a corporation of New York to obtain exactly the correct quantity for the diffusion Filed Dec. 31, 1962, Ser. No. 248,679 process. The zinc oxide on the surface of the metallic Zinc 7 Claims. (C. 48-189) diffusant material is very undesirable for a number of This invention relates to an improved diffusion process IO reasons. The oxygen is not wanted in the diffusion Vapor, for the production of Semiconductor devices, and more and the zinc oxide tends to form a protective coating particularly to an improved vapor diffusion process in over the zinc which inhibits the formation of the desired which the introduction of unwanted impurities is very zinc metal vapor which is required for the diffusion proc effectively and simply avoided, and which possesses other CSS. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

On the New Oxyarsenides Eu5zn2as5o and Eu5cd2as5o
crystals Article On the New Oxyarsenides Eu5Zn2As5O and Eu5Cd2As5O Gregory M. Darone 1,2, Sviatoslav A. Baranets 1,* and Svilen Bobev 1,* 1 Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA; [email protected] 2 The Charter School of Wilmington, Wilmington, DE 19807, USA * Correspondence: [email protected] (S.B.); [email protected] (S.A.B.); Tel.: +1-302-831-8720 (S.B. & S.A.B.) Received: 20 May 2020; Accepted: 1 June 2020; Published: 3 June 2020 Abstract: The new quaternary phases Eu5Zn2As5O and Eu5Cd2As5O have been synthesized by metal flux reactions and their structures have been established through single-crystal X-ray diffraction. Both compounds crystallize in the centrosymmetric space group Cmcm (No. 63, Z = 4; Pearson symbol oC52), with unit cell parameters a = 4.3457(11) Å, b = 20.897(5) Å, c = 13.571(3) Å; and a = 4.4597(9) Å, b = 21.112(4) Å, c = 13.848(3) Å, for Eu5Zn2As5O and Eu5Cd2As5O, respectively. The crystal structures include one-dimensional double-strands of corner-shared MAs4 tetrahedra (M = Zn, Cd) and As–As bonds that connect the tetrahedra to form pentagonal channels. Four of the five Eu atoms fill the space between the pentagonal channels and one Eu atom is contained within the channels. An isolated oxide anion O2– is located in a tetrahedral hole formed by four Eu cations. Applying the valence rules and the Zintl concept to rationalize the chemical bonding in Eu5M2As5O(M = Zn, Cd) reveals that the valence electrons can be counted as follows: 5 [Eu2+] + 2 [M2+] + 3 × × × [As3–] + 2 [As2–] + O2–, which suggests an electron-deficient configuration. -

Optical Constants of Amorphous Zinc Arsenide (Zn3as2) Via Spectroscopic Ellipsometry
Optical Constants of Amorphous Zinc Arsenide (Zn3As2) via Spectroscopic Ellipsometry James Colter Stewart A senior thesis submitted to the faculty of Brigham Young University In partial fulfillment of the requirements for the degree of Bachelor of Science John Colton, Advisor Department of Physics and Astronomy Brigham Young University April 2019 Copyright © 2019 James Colter Stewart All Rights Reserved ABSTRACT Optical Constants of Amorphous Zinc Arsenide (Zn3As2) via Spectroscopic Ellipsometry James Colter Stewart Department of Physics and Astronomy Bachelor of Science Zinc oxide (ZnO) is a promising wide band gap semiconductor with applications in ultraviolet optoelectronics. Through a novel sputtering process, our group seeks to create arsenic- doped p-type ZnO. In order to characterize these samples, we must understand the thin evaporated zinc arsenide (Zn3As2) layer in between the sputtered ZnO and our substrates. We characterize these samples by variable-angle spectroscopic ellipsometry (VASE), scanning electron microscopy (SEM), and X-ray diffraction (XRD). Enclosed is a paper our research group has submitted to Optics Express that reports the results of these characterizations; these results show that a five-parameter ellipsometric model is sufficient to find the optical constants for amorphous Zn3As2 samples. Further work needs to be done in order to properly characterize crystalline Zn3As2 samples via ellipsometry. Keywords: ellipsometry; zinc oxide; zinc arsenide; scanning electron microscopy (SEM); X-ray diffraction (XRD) ii Acknowledgments I would first like to thank my mentors Drs. John Colton and David Allred for their guidance and support of this project. Dr. Allred spent more than one Saturday with me on campus helping with the writing of the paper that comprises the bulk of this thesis. -

The University of Chicago Next Generation Synthetic
THE UNIVERSITY OF CHICAGO NEXT GENERATION SYNTHETIC ROUTES TO COLLOIDAL III-V QUANTUM DOTS A DISSERTATION SUBMITTED TO THE FACULTY OF THE DIVISION OF THE PHYSICAL SCIENCES IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF CHEMISTRY BY VISHWAS SRIVASTAVA CHICAGO, ILLINOIS AUGUST 2018 TABLE OF CONTENTS List of Figures ................................................................................................................... vii List of Tables ................................................................................................................... xiv Acknowledgments..............................................................................................................xv Abstract ........................................................................................................................... xvii CHAPTER 1: An Introduction to colloidal semiconductor nanocrystals ....................1 CHAPTER 2: Aminoarsines as arsenic precursors for the synthesis of metal arsenide nanocrystals ......................................................................................................................14 2.1 Introduction .................................................................................................................14 2.2 DIBAL-H mediated synthesis of InAs Nanocrystals ..................................................16 2.3 Effect of DIBAL-H concentration and temperature on NC size .................................18 2.4 Structural Characterization of InAs NCs ....................................................................21 -

Semiconductor and Magnetic Material 3
Semiconductor and 11 Magnetic Material 2 Electronic Devices Circuits and Applications 1.1 INTRODUCTION Semiconductors are materials having electrical conductivities between those of good conductors and insulators. Semiconductors resistivity varies from 10–5 to 10+4 m. Similarly resistivity range values 10–8 to 10+6 m for conductors and from 107 to 108 m for insulators. Germanium (Ge) and Silicon (Si) are the most commonly used semiconductors and belong to Group-IV of the periodic table. They have resistivity of about 0.6 and 1.5 × 103 m respectively. Also there are certain compound semiconductors such as gallium arsenide (GaAs), indium phosphide (InP), cadmium sulphide (CdS), etc. They are formed by the combination of the elements of groups III and V. Small band gap is another important characteristic of semiconductors. Also the semiconductors have negative temperature coefficient of resistance because the number of carriers in a semiconductor will increase significantly with temperature, resulting in reduction of the resistance of the semiconductor. 1.2 SEMICONDUCTOR MATERIALS (GROUP-IV) Semiconductors are materials having electrical conductivities between those of good conductors and insulators. The elemental semiconductor such as germanium (Ge) and silicon (Si) belong to Group-IV of the periodic table and have resistivity of about 0.6 and 1.5 × 103 cm respectively. The energy band gaps of these elements of Group-IV at 0 K are given as below: C (diamond) → 5.51 eV Ge → 0.75 eV Si → 1.16 eV Sn (grey) → 0.08 eV Pd → ≈ eV We may say from above list that at room temperature (i.e., 0 K) diamond behaves as an insulator but Ge and Si are treated as semiconductors. -

Superconductivity Induced by Hydrogen Anion Substitution in 1111-Type Iron Arsenides
Superconductivity induced by hydrogen anion substitution in 1111-type iron arsenides Hideo Hosono,1,2,3* Satoru Matsuishi3 1Materials and Structures Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan 2Frontier Research Center, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan 3Materials Research Center for Element Strategy, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan *Corresponding author: Hideo Hosono Materials and Structures Laboratory, Tokyo Institute of Technology 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan TEL +81-45-924-5359 FAX +81-45-924-5339 E-mail: [email protected] Abstract Hydrogen is the simplest bipolar element and its valence state can be controlled from +1 to −1. We synthesized the 1111-type iron arsenides CaFeAsH and LnFeAsO1−xHx (Ln = lanthanide; 0 ≤ x ≤ 0.5) with the ZrCuSiAs type structure by a high-pressure synthesis method. The position and valence state of the substituted H were determined by neutron diffraction and density functional theory calculations. The close similarity in the structural and electrical properties of CaFeAsH and CaFeAsF indicated the formation of the hydride ion (H−), which is isovalent with the fluoride ion (F−), in the 1111-type iron arsenides. When some of the O2− ions in LnFeAsO are replaced by H−, superconductivity is induced by electron doping to the FeAs-layer to maintain charge neutrality. Since the substitution limit of hydrogen in LnFeAsO (x ≈ 0.5) is much higher than that of fluorine (x ≈ 0.2), the hydrogen substitution technique provides an effective pathway for high-density electron-doping, making it possible to draw the complete electronic phase diagram of LnFeAsO. -
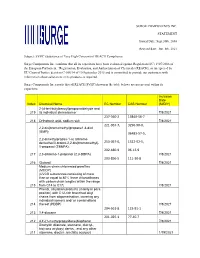
SURGE COMPONENTS INC. STATEMENT Issued Date: Sept.30Th, 2016 Revised Date: Jun. 8Th, 2021 Subject: SVHC (Substance of Very High
SURGE COMPONENTS INC. STATEMENT Issued Date: Sept.30th, 2016 Revised date: Jun. 8th, 2021 Subject: SVHC (Substance of Very High Concern) of REACH Compliance Surge Components Inc. confirms that all its capacitors have been evaluated against Regulation (EC) 1907/2006 of the European Parliament, “Registration, Evaluation, and Authorization of Chemicals (REACH), as interpreted by EU Court of Justice decision C-106/14 of 10 September 2015 and is committed to provide our customers with information about substances in its products as required. Surge Components Inc.reports that all REACH SVHC(shown in the table below) are not present within its capacitors. Inclusion Date Index Chemical Name EC Number CAS Number (M/D/Y) 2-(4-tert-butylbenzyl)propionaldehyde and 219 its individual stereoisomer 7/8/2021 237-560-2 13840-56-7 218 Orthoboric acid, sodium salt 7/8/2021 221-967-7, 3296-90-0, 2,2-bis(bromomethyl)propane1,3-diol (BMP); 36483-57-5, 2,2-dimethylpropan-1-ol, tribromo derivative/3-bromo-2,2-bis(bromomethyl)- 253-057-0, 1522-92-5, 1-propanol (TBNPA); 202-480-9 96-13-9 217 2,3-dibromo-1-propanol (2,3-DBPA) 7/8/2021 203-856-5 111-30-8 216 Glutaral 7/8/2021 Medium-chain chlorinated paraffins (MCCP) (UVCB substances consisting of more than or equal to 80% linear chloroalkanes with carbon chain lengths within the range 215 from C14 to C17) 7/8/2021 Phenol, alkylation products (mainly in para position) with C12-rich branched alkyl chains from oligomerisation, covering any individual isomers and/ or combinations 214 thereof (PDDP) 7/8/2021 204-661-8 123-91-1 213 1,4-dioxane 7/8/2021 201-025-1 77-40-7 212 4,4'-(1-methylpropylidene)bisphenol 7/8/2021 Dioctyltin dilaurate, stannane, dioctyl-, bis(coco acyloxy) derivs., and any other 211 stannane, dioctyl-, bis(fatty acyloxy) 1/19/2021 derivs. -

Solid State Physics for the Structure of Uranium Oxide and Zinc Arsenide
SOLID STATE PHYSICS FOR THE STRUCTURE OF URANIUM OXIDE AND ZINC ARSENIDE by Lydia S Harris A senior thesis submitted to the faculty of Brigham Young University - Idaho in partial fulfillment of the requirements for the degree of Bachelor of Science Department of Physics Brigham Young University - Idaho December 2019 Copyright c 2019 Lydia S Harris All Rights Reserved BRIGHAM YOUNG UNIVERSITY - IDAHO DEPARTMENT APPROVAL of a senior thesis submitted by Lydia S Harris This thesis has been reviewed by the research committee, senior thesis coor- dinator, and department chair and has been found to be satisfactory. Date Lance J Nelson, Advisor Date David Oliphant, Senior Thesis Coordinator Date Evan Hansen, Committee Member Date Todd Lines, Chair ABSTRACT SOLID STATE PHYSICS FOR THE STRUCTURE OF URANIUM OXIDE AND ZINC ARSENIDE Lydia S Harris Department of Physics Bachelor of Science Material properties are based on the structure of the material. Two ways to determine the structure of a material are a computational search for the ground state structures, and an experimental look using X-ray diffraction. In this work, these two ways are described and utilized to find the structure of two materials, uranium dioxide and zinc arsenide. Experimental techniques such as X-ray diffraction are used in order to better understand existing materials, but computational searches can be used in materials discovery. The consequences of using computational techniques is that new alloys with desirable properties can be discovered using inexpensive computer resources, however, the existence of such alloys must be validated experimentally. ACKNOWLEDGMENTS I would like to thank my parents, family, and friends for their support in my life, a.k.a. -

List of Semiconductor Materials - Wikipedia, the Free Encyclopedia Page 1 of 4
List of semiconductor materials - Wikipedia, the free encyclopedia Page 1 of 4 List of semiconductor materials From Wikipedia, the free encyclopedia Semiconductor materials are insulators at absolute zero temperature that conduct electricity in a limited way at room temperature. The defining property of a semiconductor material is that it can be doped with impurities that alter its electronic properties in a controllable way. Because of their application in devices like transistors (and therefore computers) and lasers, the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. The most commonly used semiconductor materials are crystalline inorganic solids. These materials can be classified according to the periodic table groups from which their constituent atoms come. Semiconductor materials are differing by their properties. Compound semiconductors have advantages and disadvantages in comparison with silicon. For example gallium arsenide has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allows operation of power devices at higher temperatures, and gives lower thermal noise to low power devices at room temperature; its direct band gap gives it more favorable optoelectronic properties than the indirect band gap of silicon; it can be alloyed to ternary and quaternary compositions, with adjustable band gap width, allowing light emission at chosen wavelengths, and allowing e.g. matching to wavelengths with lowest losses in optical fibers. GaAs can be also grown in a semiinsulating form, which is suitable as a lattice-matching insulating substrate for GaAs devices. Conversely, silicon is robust, cheap, and easy to process, while GaAs is brittle, expensive, and insulation layers can not be created by just growing an oxide layer; GaAs is therefore used only where silicon is not sufficient.[1] Some materials can be prepared with tunable properties, e.g. -
United States Patent 19 (11) 3,925,121 Touchy (45) Dec
United States Patent 19 (11) 3,925,121 Touchy (45) Dec. 9, 1975 54 PRODUCTION OF SEMCONDUCTIVE 3,255,056 6/1966 Flatley et al.................... 148/9 X MONOCRYSTALS OF GROUP -V 3,298,879 lf 1967 Scott et al........................... 148? 87 3,408,238 10, 1968 Sanders........................... 148/190 X SEMCONDUCTOR COMPOUNDS 3,422,322 171969 Haisty............................. 48/190 X 75) Inventor: Wolfgang Touchy, Munich, 3,502,518 3/970 Antell............................. 148/190 X Germany 3,537,921 1 / 1970 Boland............................ 48/89 X 1. y 3,660, 156 5? 1972 Schmidt.......................... 48/89 X (73) Assignee: Siemens Aktiengesellschaft, Berlin & 3,660, 78 5, 1972 Takahashi et al................... 148,189 Munich, Germany 22) Filed: Mar. 8, 1973 Primary Examiner-G. Ozaki Attorney, Agent, or Firm-Hill, Gross, Simpson, Van (21) Appl. No.: 339,218 Santen, Steadman, Chiara & Simpson (44. Published under the Trial Voluntary Protest Program on January 28, 1975 as document no. 57 ABSTRACT B 339,218. Semiconductor monocrystals composed of Group (30) Foreign Application Priority Data III-V compounds such as GaAs having a p-conductive layer therein are produced by masking a surface of a Mar. 23, 1972 Germany............................ 224224. Group III-V monocrystal with a protective layer com posed of a material, such as silicon dioxide which al (52) U.S. Cl. ................ 148/189; 1481 i 87; 148719 l; lows Group III elements to diffuse through the layer 252/62.3 GA; 357/30 while preventing diffusion of Group V elements, sub 5 Int. Cl’............................................ HOL 7/44 jecting the masked monocrystal to a heat treatment in (58) Field of Search .......... -
(12) United States Patent 20Um
USO08057654B2 (12) United States Patent (10) Patent No.: US 8,057,654 B2 M00re et al. (45) Date of Patent: Nov. 15, 2011 (54) ELECTRODEPOSITION OF DIELECTRIC 3,793,278 A 2f1974 De Bona COATINGS ON SEMCONDUCTIVE 3,928, 157 A 12/1975 Suematsu et al. 3,947.338 A 3, 1976 Jerabek et al. SUBSTRATES 3,947,339 A 3, 1976 Jerabek et al. 3,962,165 A 6, 1976 Bosso et al. (75) Inventors: Kelly L. Moore, Dunbar, PA (US); Michael J. Pawlik, Glenshaw, PA (US); (Continued) Michael G. Sandala, Pittsburgh, PA (US); Craig A. Wilson, Allison Park, PA FOREIGN PATENT DOCUMENTS (US) EP OO12463 A1 6, 1980 (73) Assignee: PPG Industries Ohio, Inc., Cleveland, OTHER PUBLICATIONS OH (US) Kohler, E. P. "An Apparatus for Determining Both the Quantity of (*) Notice: Subject to any disclaimer, the term of this Gas Evolved and the Amount of Reagent Consumed in Reactions patent is extended or adjusted under 35 with Methyl Magnesium Iodide'. J. Am. Chem. Soc., 1927, 49 (12), U.S.C. 154(b) by 345 days. 3181-3188, American Chemical Society, Washington, D.C. (21) Appl. No.: 12/405,299 Primary Examiner — Alexa Neckel (22) Filed: Mar 17, 2009 Assistant Examiner — Brian Cohen (65) Prior Publication Data (74) Attorney, Agent, or Firm — Robert P. Lenart US 2009/O236231 A1 Sep. 24, 2009 (57) ABSTRACT Related U.S. Application Data A method includes: immersing a semiconductive Substrate in (60) Provisional application No. 61/037,814, filed on Mar. an electrodeposition composition, wherein at least 20 percent 19, 2008. by weight of resin Solids in the composition is a highly cross (51) Int.