Recovery of Vegetation Structure and Species Diversity After Shifting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Literaturverzeichnis
Literaturverzeichnis Abaimov, A.P., 2010: Geographical Distribution and Ackerly, D.D., 2009: Evolution, origin and age of Genetics of Siberian Larch Species. In Osawa, A., line ages in the Californian and Mediterranean flo- Zyryanova, O.A., Matsuura, Y., Kajimoto, T. & ras. Journal of Biogeography 36, 1221–1233. Wein, R.W. (eds.), Permafrost Ecosystems. Sibe- Acocks, J.P.H., 1988: Veld Types of South Africa. 3rd rian Larch Forests. Ecological Studies 209, 41–58. Edition. Botanical Research Institute, Pretoria, Abbadie, L., Gignoux, J., Le Roux, X. & Lepage, M. 146 pp. (eds.), 2006: Lamto. Structure, Functioning, and Adam, P., 1990: Saltmarsh Ecology. Cambridge Uni- Dynamics of a Savanna Ecosystem. Ecological Stu- versity Press. Cambridge, 461 pp. dies 179, 415 pp. Adam, P., 1994: Australian Rainforests. Oxford Bio- Abbott, R.J. & Brochmann, C., 2003: History and geography Series No. 6 (Oxford University Press), evolution of the arctic flora: in the footsteps of Eric 308 pp. Hultén. Molecular Ecology 12, 299–313. Adam, P., 1994: Saltmarsh and mangrove. In Groves, Abbott, R.J. & Comes, H.P., 2004: Evolution in the R.H. (ed.), Australian Vegetation. 2nd Edition. Arctic: a phylogeographic analysis of the circu- Cambridge University Press, Melbourne, pp. marctic plant Saxifraga oppositifolia (Purple Saxi- 395–435. frage). New Phytologist 161, 211–224. Adame, M.F., Neil, D., Wright, S.F. & Lovelock, C.E., Abbott, R.J., Chapman, H.M., Crawford, R.M.M. & 2010: Sedimentation within and among mangrove Forbes, D.G., 1995: Molecular diversity and deri- forests along a gradient of geomorphological set- vations of populations of Silene acaulis and Saxi- tings. -

Kadoorie Farm and Botanic Garden, 2004. Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001
Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001 Kadoorie Farm and Botanic Garden in collaboration with Zhongshan University Zhaoqing Forestry Bureau February 2004 South China Forest Biodiversity Survey Report Series: No. 37 (Online Simplified Version) Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001 Editors Bosco P.L. Chan, Ng Sai-Chit, Michael W.N. Lau and John R. Fellowes Contributors Kadoorie Farm and Botanic Garden: Michael W.N. Lau (ML) Bosco P.L. Chan (BC) John R. Fellowes (JRF) Lee Kwok Shing (LKS) Ng Sai-Chit (NSC) Roger Kendrick (RCK) Zhongshan University: Chang Hong (CH) Voluntary specialists: Graham T. Reels (GTR) Keith D.P. Wilson (KW) Background The present report details the findings of a trip to Northwest Guangdong by members of Kadoorie Farm and Botanic Garden (KFBG) in Hong Kong and their colleagues, as part of KFBG's South China Biodiversity Conservation Programme (renamed the China Programme in 2003). The overall aim of the programme is to minimise the loss of forest biodiversity in the region, and the emphasis in the first three years is on gathering up-to-date information on the distribution and status of fauna and flora. Citation Kadoorie Farm and Botanic Garden, 2004. Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001 . South China Forest Biodiversity Survey Report Series (Online Simplified Version): No. 37. KFBG, Hong Kong SAR, ii + 33 pp. Copyright Kadoorie Farm and Botanic Garden Corporation Lam Kam Road, Tai Po, N.T., Hong Kong February 2004 - i - Contents Objectives ……………………………………………………………………………………. -

Plastome Structure and Phylogenetic Relationships of Styracaceae (Ericales)
Plastome Structure and Phylogenetic Relationships of Styracaceae (Ericales) Xiu-lian Cai Hainan University Jacob B. Landis Cornell University Hong-Xin Wang Hainan University Jian-Hua Wang Hainan University Zhi-Xin Zhu Hainan University Huafeng Wang ( [email protected] ) Hainan University https://orcid.org/0000-0002-0218-1728 Review Keywords: Styracaceae, Plastome, Genome structure, Phylogeny, positive selection Posted Date: January 29th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-55283/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/25 Abstract Background: The Styracaceae are a woody, dicotyledonous family containing 12 genera and an estimated 160 species. Recent studies have shown that Styrax and Sinojackia are monophyletic, Alniphyllum and Bruinsmia cluster into a clade with an approximately 20-kb inversion in the Large Single-Copy (LSC) region. Halesia and Pterostyrax are not supported as monophyletic, while Melliodendron and Changiostyrax always form sister clades . Perkinsiodendron and Changiostyrax were newly established genera of Styracaceae. However, the phylogenetic relationship of Styracaceae at the genera level needs further research. Results: We collected 28 complete plastomes of Styracaceae, including 12 sequences newly reported here and 16 publicly available complete plastome sequences, comprising 11 of the 12 genera of Styracaceae. All species possessed the typical quadripartite structure of angiosperm plastomes, and the sequence difference is small, except for the large 20-kb (14 genes) inversion region found in Alniphyllum and Bruinsmia. Seven coding sequences (rps4, rpl23, accD, rpoC1, psaA, rpoA and ndhH) were identied to possess positively selected sites. Phylogenetic reconstructions based on seven data sets (i.e., LSC, SSC, IR, Coding, Non-coding, combination of LSC+SSC and concatenation of LSC+SSC+one IR) produced similar topologies. -

Literature Cited
Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volume 8, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treat- ments, and also in nomenclatural citations, the titles of serials are rendered in the forms recom- mended in G. D. R. Bridson and E. R. Smith (1991). When those forms are abbreviated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and F. A. Stafleu et al. (1992– 2009). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alpha- betically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix “a”; thus, the sequence of explicit suffixes begins with “b”. There may be citations in this list that have dates suffixed “b” but that are not preceded by citations of “[a]” works for the same year, or that have dates suffixed “c,” “d,” or “e” but that are not preceded by citations of “[a],” “b,” “c,” and/or “d” works for that year. -

Ethnomedicine and Materia Medica Used by Kui Traditional Healers in Northeast Thailand
ETHNOMEDICINE AND MATERIA MEDICA USED BY KUI TRADITIONAL HEALERS IN NORTHEAST THAILAND MISS ARIKA VIRAPONGSE A THESIS FOR THE DEGREE OF MASTER OF SCIENCE KHON KAEN UNIVERSITY 2006 ISBN 974-626-847-3 ETHNOMEDICINE AND MATERIA MEDICA USED BY KUI TRADITIONAL HEALERS IN NORTHEAST THAILAND MISS ARIKA VIRAPONGSE A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN PHARMACEUTICAL CHEMISTRY AND NATURAL PRODUCTS GRADUATE SCHOOL KHON KAEN UNIVERSITY 2006 ISBN 974-626-847-3 อารีกา วีระพงส. 2549. การแพทยพื้นบานและยาสมุนไพรที่ใชโดยหมอพื้นบานชนชาติกุยใน ภาคตะวันออกเฉียงเหนือของประเทศไทย. วิทยานิพนธปริญญาวิทยาศาสตรมหาบณฑั ิต สาขาวิชาเภสชเคมั ีและผลิตภัณฑธรรมชาติ บัณฑิตวิทยาลัย มหาวิทยาลัยขอนแกน. [ISBN 974-626-847-3] อาจารยที่ปรึกษาวิทยานิพนธ: รศ.ดร. ชยันต พิเชียรสุนทร, ผศ.ดร.จุลรัตน คนศิลป, อ. ประธาน ฦาชา บทคัดยอ ชนชาติกุยเปนกลุมชาติพันธุหนึ่งในหาสิบกลุมชาติพันธุที่ไมใชไตในประเทศไทย และอาจเปน กลุมชาติพันธุแรก ๆ ที่เขามาตั้งถิ่นฐานอาศัยอยูทางตอนใตของภาคตะวันออกเฉียงเหนือของประเทศไทย การศึกษานี้มีวัตถุประสงคเพื่อศึกษาประมวลแนวคิดในเชิงทฤษฎีในระบบการแพทยพื้นบานของกลุมชาติ พันธุกุย และยาสมุนไพรที่ใชบําบัดโรคหรือความเจ็บปวยตาง ๆ กลุมประชากรที่ใชในการศึกษาครั้งนี้เปน หมอพื้นบานชาวกุยจํานวน 388 คน ซึ่งตั้งถิ่นฐานอยูในพื้นที่จังหวัดสุรินทร ศรีสะเกษ และอุบลราชธานี โดยการศึกษาเชิงลึกกับหมอพื้นบานจํานวน 30 คน วิธีวิจัยที่ใชในการเก็บรวบรวมขอมูลประกอบดวยการ ใชความจําแบบเสรี บันทึกขอมูลดานสุขภาพ การสังเกตการณโดยนักวิจัย การสังเกตการณโดยผูเขารวม โครงการวิจัย การเก็บรวบรวมตัวอยางสมุนไพร และการสัมภาษณ ผลที่ไดไดวิเคราะหและประมวลเปน -

Plastome Structure and Phylogenetic Relationships of Styracaceae
1 Plastome Structure and Phylogenetic Relationships of Styracaceae 2 (Ericales) 3 4 Xiu-lian Caia, Jacob B. Landisb , Hong-Xin Wang a, Jian-Hua Wang a, Zhi-Xin Zhu a, 5 Hua-Feng Wang a,* 6 7 aHainan Key Laboratory for Sustainable Utilization of Tropical Bioresources, School 8 of Life and Pharmaceutical Sciences, Hainan University, Haikou 570228, China 9 10 bSchool of Integrative Plant Science, Section of Plant Biology and the L.H. Bailey 11 Hortorium, Cornell University, Ithaca, NY 14850, USA 12 13 14 15 16 *Authors for correspondence: 17 Hua-Feng Wang, E-mail: [email protected]; 18 19 20 21 1 22 ABSTRACT 23 Background: The Styracaceae are a woody, dicotyledonous family containing 12 24 genera and an estimated 160 species. Recent studies have shown that Styrax is 25 monophyletic, Alniphyllum and Bruinsmia cluster into a clade with an approximately 26 20-kb inversion in the LSC. Halesia and Pterostyrax are not supported as 27 monophyletic, while Melliodendron and Changiostyrax always from a clade sister to 28 the rest of the family. However, the phylogenetic relationship of Styracaceae at the 29 level of genera remains ambiguous. 30 Results: We collected 28 complete plastomes of Styracaceae, including 12 sequences 31 newly reported here and 16 publicly available complete plastome sequences, 32 comprising 11 of the 12 genera of Styracaceae. All species possessed the typical 33 quadripartite structure of angiosperm plastomes, and the sequence difference is small, 34 except for the large 20-kb (14 genes) inversion region found in Alniphyllum and 35 Bruinsmia. Seven coding sequences (rps4, rpl23, accD, rpoC1, psaA, rpoA and ndhH) 36 were identified to possess positively selected sites. -
Phylogeny, Historical Biogeography, and Diversification of Angiosperm
Molecular Phylogenetics and Evolution 122 (2018) 59–79 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeny, historical biogeography, and diversification of angiosperm order T Ericales suggest ancient Neotropical and East Asian connections ⁎ Jeffrey P. Rosea, , Thomas J. Kleistb, Stefan D. Löfstrandc, Bryan T. Drewd, Jürg Schönenbergere, Kenneth J. Sytsmaa a Department of Botany, University of Wisconsin-Madison, 430 Lincoln Dr., Madison, WI 53706, USA b Department of Plant Biology, Carnegie Institution for Science, 260 Panama St., Stanford, CA 94305, USA c Department of Ecology, Environment and Botany, Stockholm University, SE-106 91 Stockholm Sweden d Department of Biology, University of Nebraska-Kearney, Kearney, NE 68849, USA e Department of Botany and Biodiversity Research, University of Vienna, Rennweg 14, AT-1030, Vienna, Austria ARTICLE INFO ABSTRACT Keywords: Inferring interfamilial relationships within the eudicot order Ericales has remained one of the more recalcitrant Ericaceae problems in angiosperm phylogenetics, likely due to a rapid, ancient radiation. As a result, no comprehensive Ericales time-calibrated tree or biogeographical analysis of the order has been published. Here, we elucidate phyloge- Long distance dispersal netic relationships within the order and then conduct time-dependent biogeographical and diversification Supermatrix analyses by using a taxon and locus-rich supermatrix approach on one-third of the extant species diversity -

Nghiên Cứu Khả Năng Kết Hợp Và Mức Độ Chống Chiu Sâu
ISSN: 1859-2171 TNU Journal of Science and Technology 207(14): 201 - 206 e-ISSN: 2615-9562 NGHIÊN CỨU PHÂN LOẠI CHI BỒ AN – COLONA CAV. (HỌ ĐAY – TILIACEAE JUSS.) Ở VIỆT NAM Đỗ Thị Xuyến*1, Nguyễn Anh Đức1, Ngô Thùy Linh2, Kiều Cẩm Nhung3 1Trường Đại học Khoa học Tự nhiên – ĐH QGHN; 2Trường Đại học Giáo dục - ĐHQGHN, 3Sở Giáo dục và Đào tạo Hà Nội TÓM TẮT Theo Tang Ya, Michael G. G. và Laurence J. D. (2007), chi Bồ an (Colona Cav.) có khoảng 30 loài phân bố chủ yếu ở các vùng nhiệt đới trên thế giới. Ở Việt Nam, chi này hiện biết có 9 loài là Bồ an (Colona auriculata), Chàm ron (C. evecta), Bồ an evrard (C. evrardii), Cọ mai nháp lá tròn (C. floribunda), Ko đáp (C. kodap), Nu bla (C. nubla), Cọ mai nháp lá nhỏ (C. poilanei), Bồ an nhám (C. scabra), Cọ mai nháp bốn cánh (C. thorelii). Các loài thuộc chi này chủ yếu là các loài cây gỗ, hiếm khi là cây bụi. Thường là các loài ít gặp ngoài tự nhiên, trong đó ba loài Cọ mai nháp lá nhỏ (C. poilanei), Nu bla (C. nubla), Bồ an evrard (C. evrardii) còn là các loài đặc hữu. Hầu hết các loài thuộc chi Bồ an được ghi nhận cho gỗ, 3 loài cho sợi, 1 loài được sử dụng làm thuốc. Chi Bồ an có đặc điểm hình thái gần gũi với nhiều chi thuộc họ Đay vì cùng có quả có cánh như Berrya, Craigia, Excentrodendron nhưng khác biệt bởi đặc điểm hoa lưỡng tính, có 5 cánh hoa. -

Download From
Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9 th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY . Nguyen Duc Tu DD MM YY BirdLife International – Vietnam Programme N6/2+3, Lane 25, Lang Ha St. P.O. Box 89, No. 6 Dinh Le St. Hanoi, Vietnam Designation date Site Reference Number Tel: +84 4 3514 89 04 Fax: +84 4 3514 89 21 Email: [email protected] Dr. Tran Ngoc Cuong Head of Ecology Division, Biodiversity Conservation Agency Vietnam’s Environment Protection Administration Tel: +84 4 39412025 Email: [email protected] 2. -
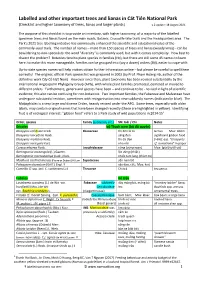
Labelled and Other Important Trees and Lianas in Cát Tiên National Park (Checklist and Higher Taxonomy of Trees, Lianas and Larger Plants) V.2 Update: 18 August 2021
Labelled and other important trees and lianas in Cát Tiên National Park (Checklist and higher taxonomy of trees, lianas and larger plants) v.2 update: 18 August 2021 The purpose of this checklist is to provide an inventory, with higher taxonomy, of a majority of the labelled specimen trees and lianas found on the main roads, Botanic, Crocodile-lake trails and the Headquarters area. The Park's 2021 tree labelling initiative has enormously enhanced the scientific and educational value of the commonly-used trails. The number of names – more than 150 species of trees and lianas (woody vines) - can be bewildering to non-specialists: the word "diversity" is commonly used, but with it comes complexity. How best to dissect the problem? Botanists tend to place species in families (Họ), but there are still some 45 names to learn here; to make this more manageable, families can be grouped into (say a dozen) orders (Bộ), easier to cope-with. Up-to-date species names will help visitors obtain further information online – but please be careful to spell them correctly! The original, official Park species list was prepared in 2002 (by Prof. Phạm Hoàng Hộ, author of the definitive work Cây Cỏ Việt Nam). However since then, plant taxonomy has been revised substantially by the international Angiosperm Phylogeny Group (APG), with whole plant families promoted, demoted or moved to different orders. Furthermore, genera and species have been – and continue to be - revised in light of scientific evidence; this also can be confusing for non-botanists. Two important families, the Fabaceae and Malvaceae have undergone substantial revision, sometimes with reorganisation into new subfamily names (indicated in blue). -

Plastome Structure and Phylogenetic Relationships of Styracaceae (Ericales) Xiu‑Lian Cai1, Jacob B
Cai et al. BMC Ecol Evo (2021) 21:103 BMC Ecology and Evolution https://doi.org/10.1186/s12862-021-01827-4 RESEARCH ARTICLE Open Access Plastome structure and phylogenetic relationships of Styracaceae (Ericales) Xiu‑Lian Cai1, Jacob B. Landis2,3, Hong‑Xin Wang1, Jian‑Hua Wang1, Zhi‑Xin Zhu1 and Hua‑Feng Wang1* Abstract Background: The Styracaceae are a woody, dicotyledonous family containing 12 genera and an estimated 160 spe‑ cies. Recent studies have shown that Styrax and Sinojackia are monophyletic, Alniphyllum and Bruinsmia cluster into a clade with an approximately 20‑kb inversion in the Large Single‑Copy (LSC) region. Halesia and Pterostyrax are not supported as monophyletic, while Melliodendron and Changiostyrax always form sister clades. Perkinsiodendron and Changiostyrax are newly established genera of Styracaceae. However, the phylogenetic relationship of Styracaceae at the generic level needs further research. Results: We collected 28 complete plastomes of Styracaceae, including 12 sequences newly reported here and 16 publicly available sequences, comprising 11 of the 12 genera of Styracaceae. All species possessed the typical quadri‑ partite structure of angiosperm plastomes, with sequence diferences being minor, except for a large 20‑kb (14 genes) inversion found in Alniphyllum and Bruinsmia. Seven coding sequences (rps4, rpl23, accD, rpoC1, psaA, rpoA and ndhH) were identifed to possess positively selected sites. Phylogenetic reconstructions based on seven data sets (i.e., LSC, SSC, IR, Coding, Non‑coding, combination of LSC SSC and concatenation of LSC SSC one IR) produced similar topologies. In our analyses, all genera were strongly+ supported as monophyletic. Styrax+ was+ sister to the remaining genera. -

Nghiên Cứu Đa Dạng Thực Vật Bậc Cao Có Mạch Và Đề Xuất Các Giải Pháp Bảo Tồn Ở Khu Bảo Tồn Thiên Nhiên Pù Hoạt, Tỉnh Nghệ An
BỘ GIÁO DỤC VÀ ĐÀO TẠO VIỆN HÀN LÂM KHOA HỌC VÀ CÔNG NGHỆ VIỆT NAM HỌC VIỆN KHOA HỌC VÀ CÔNG NGHỆ ----------------------------- NGUYỄN DANH HÙNG NGHIÊN CỨU ĐA DẠNG THỰC VẬT BẬC CAO CÓ MẠCH VÀ ĐỀ XUẤT CÁC GIẢI PHÁP BẢO TỒN Ở KHU BẢO TỒN THIÊN NHIÊN PÙ HOẠT, TỈNH NGHỆ AN LUẬN ÁN TIẾN SỸ SINH HỌC HÀ NỘI - 2020 VIỆN HÀN LÂM KHOA HỌC VÀ CÔNG NGHỆ VIỆT NAM HỌC VIỆN KHOA HỌC VÀ CÔNG NGHỆ ……..….***………… NGUYỄN DANH HÙNG NGHIÊN CỨU ĐA DẠNG THỰC VẬT BẬC CAO CÓ MẠCH VÀ ĐỀ XUẤT CÁC GIẢI PHÁP BẢO TỒN Ở KHU BẢO TỒN THIÊN NHIÊN PÙ HOẠT, TỈNH NGHỆ AN LUẬN ÁN TIẾN SỸ SINH HỌC Chuyên ngành: Thực vật học Mã số: 9.42.01.11 Người hướng dẫn khoa học: 1. TS. Đỗ Ngọc Đài 2. PGS. TS. Trần Minh Hợi Hà Nội - 2020 LỜI CAM ĐOAN Tôi xin cam đoan, đây là công trình nghiên cứu riêng tôi. Các số liệu trong luận án được trích dẫn đầy đủ, rõ ràng. Tác giả luận án NCS Nguyễn Danh Hùng MỤC LỤC MỞ ĐẦU ...................................................................................................... 1 1. Tính cấp thiết của đề tài luận án ............................................................... 1 2. Mục tiêu nghiên cứu ................................................................................. 2 3. Ý nghĩa khoa học và thực tiễn .................................................................. 2 4. Bố cục của luận án .................................................................................... 2 CHƯƠNG 1. TỔNG QUAN TÀI LIỆU....................................................... 3 1.1. Đa dạng sinh học .................................................................................... 3 1.2. Nghiên cứu về thực vật trên thế giới ..................................................... 3 1.2.1. Nghiên cứu về hệ thực vật .................................................................. 3 1.2.2.