ED149972.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Maintenance of Bacterial Strains
Maintenance of Bacterial Strains Short-term growth and maintenance The simplest method for obtaining strains is to order them from a microbiological supply company. Presque Isle, Ward, and Carolina Biological (see “Resources” page of the Microbes Web site: www.umsl.edu/~microbes) are economical sources of strains that are commonly used in the classroom. Another possible source is a local college or university that teaches a microbiology lab course. If they have strains already prepared for their class, they will probably be willing to provide you with a culture. This is probably not a reasonable ongoing source of strains, but can be useful in an emergency. For short-term maintenance and use, it is best to streak bacteria on some type of rich agar petri plate, such as nutrient agar. Label and date the plate on the bottom of the plate. Streak from the source culture using good sterile technique. The quadrant streak method (see the article on quadrant streaking) is good for obtaining well-isolated colonies. Incubate the plate only until good colonies have formed. Do not leave the culture in the incubator beyond that time, or the cells will die on the plate. Store the plate in the refrigerator. It will keep for about a month. For class use, it is best to streak a fresh plate 2-3 days before the strain will be needed for class. Use the fresh plate as the source of cultures for the class. For broth cultures for a class, it is best to grow a larger volume culture in a flask (if possible, with shaking) overnight and then dispense aliquots of the master culture into sterile test tubes using a sterile pipette. -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

Laboratory Supplies and Equipment
Laboratory Supplies and Equipment Beakers: 9 - 12 • Beakers with Handles • Printed Square Ratio Beakers • Griffin Style Molded Beakers • Tapered PP, PMP & PTFE Beakers • Heatable PTFE Beakers Bottles: 17 - 32 • Plastic Laboratory Bottles • Rectangular & Square Bottles Heatable PTFE Beakers Page 12 • Tamper Evident Plastic Bottles • Concertina Collapsible Bottle • Plastic Dispensing Bottles NEW Straight-Side Containers • Plastic Wash Bottles PETE with White PP Closures • PTFE Bottle Pourers Page 39 Containers: 38 - 42 • Screw Cap Plastic Jars & Containers • Snap Cap Plastic Jars & Containers • Hinged Lid Plastic Containers • Dispensing Plastic Containers • Graduated Plastic Containers • Disposable Plastic Containers Cylinders: 45 - 48 • Clear Plastic Cylinder, PMP • Translucent Plastic Cylinder, PP • Short Form Plastic Cylinder, PP • Four Liter Plastic Cylinder, PP NEW Polycarbonate Graduated Bottles with PP Closures Page 21 • Certified Plastic Cylinder, PMP • Hydrometer Jar, PP • Conical Shape Plastic Cylinder, PP Disposal Boxes: 54 - 55 • Bio-bin Waste Disposal Containers • Glass Disposal Boxes • Burn-upTM Bins • Plastic Recycling Boxes • Non-Hazardous Disposal Boxes Printed Cylinders Page 47 Drying Racks: 55 - 56 • Kartell Plastic Drying Rack, High Impact PS • Dynalon Mega-Peg Plastic Drying Rack • Azlon Epoxy Coated Drying Rack • Plastic Draining Baskets • Custom Size Drying Racks Available Burn-upTM Bins Page 54 Dynalon® Labware Table of Contents and Introduction ® Dynalon Labware, a leading wholesaler of plastic lab supplies throughout -

Qualitube™ Kit for Microcystin
™ QualiTube Kit For Microcystin Highlights: Catalog Number ET 022 Semi-quantitative field screening Intended Use of Microcystin toxin in surface water The EnviroLogix QualiTube Kit for Microcystin is designed for semi- Detects from 0.5 to 3 ppb quantitative field screening of Microcystin toxin in surface water samples. The kit is supplied with calibrators at 0.5 and 3 ppb. The assay range can easily be extended. How the Test Works Contents of Kit: The QualiTube Kit for Microcystin is a competitive Enzyme-Linked ImmunoSorbent Assay (ELISA). In the test, Microcystin toxin in the sample 36 antibody-coated test tubes competes with enzyme (horseradish peroxidase)-labeled Microcystin for a 1 vial of 0.5 ppb Microcystin LR limited number of antibody binding sites on the inside surface of the test Calibrator tubes. 1 vial of 3 ppb Microcystin LR Calibrator After a simple wash step, the outcome of the competition is visualized with 1 dropper bottle of Assay Diluent a color development step. As with all competitive immunoassays, sample 1 dropper bottle of Microcystin- concentration is inversely proportional to color development. enzyme Conjugate 1 dropper bottle of Substrate Darker color = Lower concentration 1 bottle of Stop Solution Lighter color = Higher concentration 36 sample pipettes Limit of Detection The Limit of Detection (LOD) of the EnviroLogix Microcystin Tube Kit is Optional Accessory Item: 0.3 ppb. The LOD was determined by interpolation at 81.5% B0* from a ACC 062 – 1 vial of 1.5 ppb standard curve. 81.5% B0 was determined to be 2 standard deviations from Microcystin LR Calibrator the mean of a population of negative water samples. -

A Passive Membrane Photobioreactor for the Isolated Cultivation of Algal
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 10-31-2014 A Passive Membrane Photobioreactor for the Isolated Cultivation of Algal Resource Utilizing Selectivity (ICARUS), with Wastewater as a Feedstock Ivy Lea Cormier Drexler University of South Florida, [email protected] Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the Civil and Environmental Engineering Commons Scholar Commons Citation Drexler, Ivy Lea Cormier, "A Passive Membrane Photobioreactor for the Isolated Cultivation of Algal Resource Utilizing Selectivity (ICARUS), with Wastewater as a Feedstock" (2014). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/5414 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. A Passive Membrane Photobioreactor for the Isolated Cultivation of Algal Resource Utilizing Selectivity (ICARUS), with Wastewater as a Feedstock by Ivy L.C. Drexler A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Engineering Science Department of Civil and Environmental Engineering College of Engineering University of South Florida Co-Major Professor: Daniel H. Yeh, Ph.D. Co-Major Professor: Norma Alcantar, Ph.D. Sarina Ergas, Ph.D. Valerie Harwood, Ph.D. Piet Lens, Ph.D. Date of Approval: October 31, 2014 Keywords: biofuel, dialysis, nutrients, phycoprospecting, phycoremediation Copyright © 2014, Ivy L.C. Drexler Dedication To Mike, my husband, The smartest person I know. And our little babe. -

Biotechnology Science for the New Millennium by Ellyn Daugherty Cheese Production: the Evolution of Cheese-Making Technology (Lab 1C)
G-Biosciences ♦ 1-800-628-7730 ♦ 1-314-991-6034 ♦ [email protected] A Geno Technology, Inc. (USA) brand name Biotechnology Science for the New Millennium by Ellyn Daugherty Cheese Production: The Evolution of Cheese-Making Technology (Lab 1c) (Cat. # BTNM-1C) w Developed in partnership with think proteins! think G-Biosciences www.GBiosciences.com Biotechnology: Science for the New Millennium by Ellyn Daugherty Cheese Production: The Evolution of Cheese-Making Technology (Lab 1c) Teacher’s Guide Page 2 of 12 Biotechnology: Science for the New Millennium by Ellyn Daugherty Cheese Production: The Evolution of Cheese-Making Technology (Lab 1c) Teacher’s Guide The following laboratory activity is adapted from “Laboratory 1c: Cheese Production: The Evolution of Cheese-Making Technology” from Biotechnology: Laboratory Manual by Ellyn Daugherty. For more information about the program, please visit www.emcp.com/biotechnology. This kit is produced under license from Paradigm Publishing, Inc., a division of New Mountain Learning. About Ellyn Daugherty: Ellyn Daugherty is a veteran biotechnology educator and recipient of the Biotechnology Institute’s National Biotechnology Teacher-Leader Award. She is the founder of the San Mateo Biotechnology Career Pathway (SMBCP). Started in 1993, SMBCP has instructed more than 10,000 high school and adult students. Annually, 30-40 SMBCP students complete internships with mentors at local biotechnology facilities. About G-Biosciences: In addition to the Biotechnology by Ellyn Daugherty laboratory kit line and recognizing the significance and challenges of life sciences education, G-Biosciences has initiated the BioScience Excellence™ program. The program features hands-on teaching kits based on inquiry and curiosity that explore the fundamentals of life sciences and relate the techniques to the real world around us. -

Physicians Office Labs
® Select products for Physicians Office Labs www.globescientific.com ® Select products for Physicians Office Labs Table of Contents E.S.R. Systems .....................................2-5 Sedi-Rate™ Starter Kit Blood Collection ................................... 6-7 Capillary Tubes .....................................8-9 Specimen Containers .......................10-13 Thermometer Strips ...............................11 Patient Identification Labels ...................11 Specimen Transport Tubes .................. 13 Specimen Transport Bags .................... 14 Urinalysis Products ............................15-17 Centrifuge Tubes ..............................18-20 3467 — Sedi-Rate Starter Kit Test Tubes .........................................21-22 Sedi-Rate™ Starter Kit includes everything needed to perform safe and Caps for Test Tubes ....................6, 23-24 accurate Westergren ESR determinations. Kit includes: Racks for Test Tubes ...................... 25-27 100 Sedi-Rate pipettes and vials 100 Piercing funnels Microscope Slides/Accessories ....28-31 100 ESR transfer pipettes Pipettors ...........................................32-33 1 10-place rack with pipette support clamps 1 Leveling platform Pipette Tips ............................................. 34 Item # Description Unit 3467 Sedi-Rate Starter Kit 1 Kit Transfer Pipets ....................................... 35 2 Customer Service and Technical Support: 1 (201) 599-1400 Sedi-Rate™ E.S.R. System The use of Sedi-Rate™ eliminates splashing, spraying and aerosol -

Energy Resources: Where Are They and How Do We Get Them?
Energy Resources: Where Are They and How Do We Get Them? SPN LESSON #5 TEACHER INFORMATION LEARNING OUTCOME: Students describe some of the many ways solar energy is converted into other forms of energy; the patterns of distribution of energy resources in the United States; and how these patterns of distribution are represented through maps. LESSON OVERVIEW: In this lesson, emphasis is on the development of interpretive skills and the use of models to reveal scientific processes. Students learn the nature of various energy resources, how they form, and the science that allows them to be discovered and extracted. Through short laboratory tasks at a series of stations, and through pencil-and-paper activities, solar, wind, water, and fossil fuel (coal) energy sources are explored. The lesson includes study of: • field maps and isolines and the information they convey; • energy conversions of sunlight into other forms of energy; • subsurface rock structures; and • why energy resources are unevenly distributed. GRADE-LEVEL APPROPRIATENESS: This Level II general energy lesson, intended for younger students, is most appropriate for grades 5–8. MATERIALS (materials needed at each station) Station 1: Coal Power Hand lenses (4) Rock specimens: • Silty shale with marine fossils [label “A”] • Limestone with marine fossils [label “D”] • Coal – bituminous with land plant fossils [label “B”] • Sandy shale with land plant fossils [label “A”] • Sandstone with land plant fossils [label “E”] Colored pencils nyserda.ny.gov/School-Power-Naturally Station 2: -
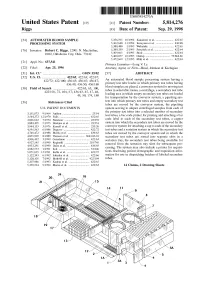
E. E. E." M. Cessing. the System May Also Include a Printer for Requisi
USOO5814276A United States Patent (19) 11 Patent Number: 5,814,276 Riggs (45) Date of Patent: Sep. 29, 1998 54 AUTOMATED BLOOD SAMPLE 5,356,595. 10/1994 Kanamori et al. ........................ 422/63 PROCESSING SYSTEM 5,362,648 11/1994 Koreyasu et al. .. ... 436/48 5,380,488 1/1995 Wakatake ........... ... 422/63 76 Inventor: Robert C. Riggs, 12901 N. MacArthur, 2 - - - 2 3.1. EtheklT0 . et. al.. .- -. .- : #102, Oklahoma City, Okla. 73142 5,460,057 10/1995 Ostrup .................................. 73/86481 21 Appl. No.: 637,541 5,472,669 12/1995 Miki et al. ................................ 422/63 TNO. 21 pp 9 Primary Examiner Long V. Le 22 Filed: Apr. 25, 1996 Attorney, Agent, or Firm-Head, Johnson & Kachigian (51) Int. Cl." ..................................................... G01N 35/02 57 ABSTRACT 52)52 U.S. Cl. .................................422/72; 422/100; it's422765: 422/63:i. 422/67:i. An automated blood Sample processing System having a s 436/49, 436750, 4361so primary test tube loader in which primary test tubes having s s blood Samples are placed, a conveyor System for moving test 58) Field of Soi 7, 1867, 437, 1. tubes in Selectable routes, a centrifuge, a Secondary test tube s as s s/ s 49 50 74 180 loading area in which empty Secondary test tubes are loaded s a Yu’s s for transportation by the conveyor System, a pipetting Sys 56) References Cited tem into which primary test tubes and empty Secondary test tubes are moved by the conveyor System, the pipetting U.S. PATENT DOCUMENTS System Serving to aliquot centrifuged Samples from each of 3,151,073 9/1964 Anthon . -

Accessories for New Brunswick™ Shakers: Dimensions and Capacities
Accessories for New Brunswick™ Shakers: Dimensions and Capacities Applications Training Document, Apr. 2014 For internal and external use Author Technical Applications, Enfield, USA Contact RSCs: Contact your local Level 2 Application Support Team Level 2 Support Teams: Contact Enfield Technical Applications Team Eppendorf, Inc. 175 Freshwater Boulevard Tel.: +1 860 253 3400 Enfield, CT 06082-4444, USA [email protected] www.eppendorf.com Table of Contents 1. Platform Dimensions ....................................................................................................................................................................3 2. Test Tube Racks ...........................................................................................................................................................................4 3. Flask Clamps ................................................................................................................................................................................5 4. Other Accessories ........................................................................................................................................................................6 www.eppendorf.com 1. Platform Dimensions This table gives the platform dimensions (in inches and centimeters) of all New Brunswick shaker models. Model Dimensions (cm) Dimensions (in) CO2 Incubator Shaker S41i 61 x 36 24 x 14 Innova® Incubated/Refrigerated 40/40R 46 x 46 18 x 18 42/42R 46 x 46 18 x 18 43/43R 76 x 46 30 x 18 44/44R 76 x 46 30 x 18 I Series I26/I26R 76 x 46 30 x 18 Excella® Incubated/Refrigerated E24/E24R 46 x 46 18 x 18 E25/E25R 76 x 46 30 x 18 Innova® Open-Air 2000 33 x 28 13 x 11 2050 41 x 31 16 x 12 2100 46 x 46 18 x 18 2150 61 x 46 24 x 18 2300 76 x 46 30 x 18 2350 91.4 x 61 36 x 24 5000 82.5 x 68 (up to 6) 32.5 x 26.7 (up to 6) Excella® Open-Air E1 33 x 28 13 x 11 E2 33 x 28 13 x 11 E5 46 x 46 18 x 18 E10 76 x 46 30 x 18 Water Bath Innova® 3100 30.5 x 42 16 x 12 C76 30.5 x 42 16 x 12 www.eppendorf.com 2. -

2016-HTW-Catalog Lo-Res.Pdf
table of contents mixing and centrifugation 4-9 tube selection guide / tube racks 10-27 tube storage 28-35 pipetting 36-43 microscopy and histology 44-49 sample and blood collection 50-59 cryo 60-71 weighing 72-75 safety 76-81 microbiology 82-85 storage 86-93 sealing and labeling 94-99 notebooks and timers 100-102 index 103 product page reference 104-105 charts and reference 106-107 True North ® lab essentials are manufactured Droplet™ sample collection products are designed specifically to withstand extremely low temperatures to help improve the quality of day-to-day work-flow for cryogenics studies. through every stage. With a comprehensive product offering for working, From sample collection, process analysis, transport labeling, transporting and storing of cryogenic and storage we offer a variety of solutions that materials, True North® products allow lab professionals provide efficiency and safety while protecting against to concentrate on their work without worrying about contamination (pages 50-59). compromising valuable samples (pages 60-71). mixing and centrifugation High-quality instruments, with advanced features for a vast range of protocols. mixing and centrifugation 5 mini centrifuge Sprout® Mini-Centrifuge SnapSpin™ rotors for easy and fast exchange The Sprout® is ideal for quick spin downs, micro-filter cell separations and HPLC samples. It is compact, easy to use and comes with a host of extras. - Small footprint and quiet operation - Palm-shaped lid, suitable for left or right handed users allows quick extraction of tubes - Snapspin™ rotors are tool-free for a fast and easy exchange - Weighted rubberized base eliminates potential movement and further dampens vibration and noise - Suitable for worldwide voltages 110 to 240 V, supplied with 4 different plug adapters Part No. -

Extreme Environment Shakers
Extreme Environment Shakers High Performance Orbital Shaker Designed to Withstand Extreme Environments Up To 100% Humidity OHAUS Extreme Environment Shakers are designed for use in applications that require CO2 and humidity for optimal cell growth. The remote controller magnetically attaches to the outside of most incubators or easily sits on the lab bench. The shaker base is placed inside the incubator and connected to the touchpad controller with a 170 cm cable allowing for simple external control of speed and time settings. This shaker features an IP65-rated motor. Standard Features: • Defy the elements - rely on this orbital shaker’s unique construction to deliver exceptional speed control, accuracy and durability under the most extreme environmental conditions. • Best-in-class load capacity to maximize efficiency in your incubator. Customize the Extreme Environment Shaker for your unique application with over 50 accessories. • For repeatable results, the exclusive Accu-Drive shaking system ensures speed accuracy. The triple eccentric drive provides smooth, stable shaking motion throughout the entire speed range. Find Quality Products Online at: www.GlobalTestSupply.com [email protected] Specifications Extreme Environment Shakers Model SHEX1619DG Control Digital Motion Orbital, 0.8 in. / 19 mm Speed Range 15 rpm – 500 rpm Speed Accuracy ± 1 rpm, Above 100 rpm ± 1% of set speed Timer 1 second – 160 hours Capacity 16 kg Working Environment Temperature (Shaker): -10 to 60 °C (14 to 140 °F), maximum 100 % relative humidity, condensing Temperature (Controller): -10 to 50 °C (14 to 122 °F), maximum 80 % relative humidity, non-condensing Drive System Brushless DC Motor; Triple Eccentric Communication RS232 (Included) Tray Size (L × W) 11 in.