Contamination of Surface Waters by Mining Wastes in the Milluni Valley (Cordillera Real, Bolivia): Mineralogical and Hydrological Influences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Línea Base De Conocimientos Sobre Los Recursos Hidrológicos E Hidrobiológicos En El Sistema TDPS Con Enfoque En La Cuenca Del Lago Titicaca ©Roberthofstede
Línea base de conocimientos sobre los recursos hidrológicos e hidrobiológicos en el sistema TDPS con enfoque en la cuenca del Lago Titicaca ©RobertHofstede Oficina Regional para América del Sur La designación de entidades geográficas y la presentación del material en esta publicación no implican la expresión de ninguna opinión por parte de la UICN respecto a la condición jurídica de ningún país, territorio o área, o de sus autoridades, o referente a la delimitación de sus fronteras y límites. Los puntos de vista que se expresan en esta publicación no reflejan necesariamente los de la UICN. Publicado por: UICN, Quito, Ecuador IRD Institut de Recherche pour Le Développement. Derechos reservados: © 2014 Unión Internacional para la Conservación de la Naturaleza y de los Recursos Naturales. Se autoriza la reproducción de esta publicación con fines educativos y otros fines no comerciales sin permiso escrito previo de parte de quien detenta los derechos de autor con tal de que se mencione la fuente. Se prohíbe reproducir esta publicación para venderla o para otros fines comerciales sin permiso escrito previo de quien detenta los derechos de autor. Con el auspicio de: Con la colaboración de: UMSA – Universidad UMSS – Universidad Mayor de San André Mayor de San Simón, La Paz, Bolivia Cochabamba, Bolivia Citación: M. Pouilly; X. Lazzaro; D. Point; M. Aguirre (2014). Línea base de conocimientos sobre los recursos hidrológicos en el sistema TDPS con enfoque en la cuenca del Lago Titicaca. IRD - UICN, Quito, Ecuador. 320 pp. Revisión: Philippe Vauchel (IRD), Bernard Francou (IRD), Jorge Molina (UMSA), François Marie Gibon (IRD). Editores: UICN–Mario Aguirre; IRD–Marc Pouilly, Xavier Lazzaro & DavidPoint Portada: Robert Hosfstede Impresión: Talleres Gráficos PÉREZ , [email protected] Depósito Legal: nº 4‐1-196-14PO, La Paz, Bolivia ISBN: nº978‐99974-41-84-3 Disponible en: www.uicn.org/sur Recursos hidrológicos e hidrobiológicos del sistema TDPS Prólogo Trabajando por el Lago Más… El lago Titicaca es único en el mundo. -

Oatao.Univ-Toulouse.Fr/ Eprints ID: 5602
Open Archive Toulouse Archive Ouverte (OATAO) OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible. This is an author-deposited version published in: http://oatao.univ-toulouse.fr/ Eprints ID: 5602 To cite this version: Salvarredy-Aranguren, Matias Miguel and Probst, Anne and Roulet, Marc Evidencias sedimentarias y geoquimicas de la pequeño edad de hielo en el lago milluni grande del altiplano boliviano. (2009) Revista de la Asociación Geológica Argentina, vol. 65 (n°4). pp. 660-673. ISSN 1851-8249 Any correspondence concerning this service should be sent to the repository administrator: [email protected] EVIDENCIAS SEDIMENTARIAS Y GEOQUIMICAS DE LA PEQUEÑA EDAD DE HIELO EN EL LAGO MILLUNI GRANDE DEL ALTIPLANO BOLIVIANO Matías Miguel SALVARREDY-ARANGUREN a,b,1* ¤ , Anne PROBST c,d y Marc ROULET a,e,† a Laboratoire des Mécanismes et Transferts en Géologie (LMTG), Toulouse, Francia. Email: [email protected] b Instituto de Geología y Recursos Minerales, Servicio Geológico Minero Argentino, Buenos Aires. c Université de Toulouse , UPS, INP , EcoLab (Laboratoire d'écologie fonctionnelle), Castanet-Tolosan, France d CNRS , EcoLab, Castanet-Tolosan, France, Email:[email protected] e Institut de Recherche pour le Développement, HYBAM, La Paz, Bolivia† † fallecido en 2006 RESUMEN El lago de Milluni Grande (LMG) está ubicado en el flanco occidental de los Andes Orientales en el valle de Milluni que po- see una clásica forma en U dado que se encuentra en una región glacial. El lago es el más grande de este valle, y dado que se halla situado al cierre de cuenca, resulta estratégico para albergar un registro sedimentario de los últimos 450 años. -

20150331191630 0.Pdf
2 3 Esta publicación cuenta con el auspicio del Fondo Climático de Ministerio de Relaciones Exteriores de Alemania y de la Embajada de la República Federal de Alemania en Bolivia. Glaciares Bolivia Testigos del 12 cambio climático Concepto, textos y edición general: Teresa Torres-Heuchel Edición gráfica:Gabriela Fajardo E. Diseño de portada y diagramación: Erik Rodríguez Archivo gráfico y documentación: Heidi Stache, Ekkehard Jordan, Deutscher Alpenverein (DAV), Instituto Boliviano de la Montaña (BMI) Fotografía de portada: Dirk Hoffmann Colaboradoras: Alicia de Mier, Johanna Hömberg El contenido de la presente publicación es de responsabilidad del Instituto Boliviano de la Montaña (BMI) Urbanización La Barqueta, Achumani Calle 28 B esquina calle 4 C Sajama 5 Teléfono: 2.71.24.32. Correo electrónico: [email protected] Casilla: 3-12417 La Paz, Bolivia Depósito Legal: 4–1–2654–14 Impreso por CREATIVA 2 488 588 (La Paz) 4 5 Vista de Mina Pacuni, Quimsa Cruz (BMI / 2014) 6 Glaciares Bolivia Testigos del 12 cambio climático Indice Prólogo Presentación 12 Glaciares I. Sajama II. San Enrique III. Illimani IV. Picacho Kasiri V. Wila Manquilisani VI. Chacaltaya VII. Chiar Kerini VIII. Zongo IX. Lengua Quebrada X. Maria Lloco XI. Wila Llojeta XII. Culin Thojo La vida en torno al glaciar Glaciares y el ciclo hídrico Bofedales, tesoros de montaña Lagunas glaciares y los nuevos riesgos para la población de montaña El cambio climático en Bolivia Bolivia y los bolivianos en el cambio climático Buscando limitar el calentamiento global a 2º C COP 20 en puertas, glaciares andinos expuestos a ojos del mundo 7 8 Región Nigruni, Cordillera Oriental (BMI / 2014) Prólogo Frenar el calentamiento global y desarrollar soluciones para la adaptación al cambio climático es una tarea global. -

The Cordillera Real
44 THE CORDILLERA REAL THE CORDILLERA REAL , BY EVELIO ECHEVARRIA C. HE lovely ranges of Southern Peru slope gradually down to the shores of Lake Titicaca, but this is not their end; to the east of Carabaya knot springs another ridge, which enters Bolivia and reaches its maximum elevation and magnificence in the Cordillera Real of the latter country. The name Cordillera Real was given by the Spaniards as homage to a range of royal dignity; it is located in the north-west of Bolivia, between I5° 40" and 16° 40" S., running roughly north-west to south east for an approximate length of 100 miles. This range was termed by Austrian mountaineers ' der Himalaya der N euen Welt'. Although this honour may now be disputed by several Peruvian cordilleras, it contains nevertheless mountain scenery of Himalayan grandeur; six twenty-thousanders and scores of lesser peaks are found in the region between Illampu (20,873 ft.) and Illimani (21 ,201 ft.), the mighty pillars of each extreme. The Cordillera Real is a snow and ice range; it forms a lovely back ground for that remarkable high plateau, the Bolivian Altiplano, and is in full sight nine months a year. The white peaks, the steppe-like plain and the empty, blue skies have given to this part of Bolivia a Tibetan air that many travellers have noticed; and the Mongolian features of the Aymara Indians, stolidly facing the chill winds, reinforce this opinion. General description In the north and in the south the Cordillera Real rises over deep mountain basins ; peaks like Illimani soar well above the wooded hills of Coroico and Inquisive, towns only 4,700 ft. -

Condoriri Laguna Chiar Khota Cumbre Huayna Potosí
Condoriri Laguna Chiar Khota Cumbre Huayna Potosí Pocas ciudades provocan sensaciones tan extraordinarias como La Paz. Rodeada por la Cordillera de Los Andes y teniendo al Illimani como su montaña tutelar, encierra atractivos naturales tan imponentes y contrastantes como el Valle de la Luna, el Valle de Zongo, con sus siete diferentes pisos ecológicos y las lagunas de Pampalarama, al pie del nevado Wilamankilisani en las que nace, cristalino, el rio Choqueyapu. Esta Guía de Montañas ofrece al turista de aventura, la posibilidad de admirar el paisaje maravilloso de la Cordillera de los Andes y enfrentar, si desea, el desafío de escalar sus picos más altos, una prueba en que el hombre se propone vencer los obstáculos de la naturaleza, quizás con el secreto afán de encontrar sus propios límites y posibilidades. La guía invita a propios y visitantes a recorrer los espectaculares paseos que ofrece la Cordillera, para la práctica del trekking, escalada en roca, alpinismo, camping, safari fotográfico, desde donde se aprecia la naturaleza multifacética y de grandes retos. Bienvenidos sean todos a conocer o a redescubrir la magia que surge del espléndido entorno natural que circunda a la ciudad de La Paz. Luis Revilla Herrero Alcalde de La Paz HUAYNA POTOSÍ 05 LAGUNA MAMANCOTA 06 TREKKING COHONI BASE CAMP 20 GLACIAR VIEJO 06 TREKKING UNNI - PINAYA - COHONI - TAHUAPALCA (PEQUEÑO ILLIMANI) 1 2 CHARQUINI 06 TREKKING COHONI - LAMBATE (GRAN ILLIMANI) 21 LAKE MAMANCOTA 07 TREKKING PINAYA - COHONI - TAHUAPALCA (SMALL ILLIMANI) 22 THE OLD GLACIER 07 TREKKING -
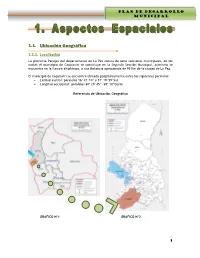
1.1. Ubicación Geográfica
PLAN DE DESARROLLO MUNICIPAL G.M. CAQUIAVIRI 1.1. Ubicación Geográfica 1.1.1. Localización La provincia Pacajes del departamento de La Paz consta de ocho secciones municipales, de los cuales el municipio de Caquiaviri se constituye en la Segunda Sección Municipal, asimismo se encuentra en la llanura altiplánica, a una distancia aproximada de 95 Km de la ciudad de La Paz. El municipio de Caquiaviri se encuentra ubicado geográficamente entre los siguientes paralelos: Latitud austral: paralelos 16º 47´10” y 17º 19´59”Sur Longitud occidental: paralelos 68º 29´45”- 69º 10”Oeste Referencia de Ubicación Geográfica GRAFICO Nº1 GRAFICO Nº2 1 PLAN DE DESARROLLO MUNICIPAL G.M. CAQUIAVIRI Ubicación del Municipio Caquiaviri en la Provincia Pacajes GRAFICO Nº3 1.1.2. Límites Territoriales El municipio de Caquiaviri tiene la siguiente delimitación: Límite Norte; Municipios de Jesús de Machaca y San Andrés de Machaca de la provincia Ingavi y el Municipio Nazacara de Pacajes que es la Séptima Sección de la provincia Pacajes del departamento de La Paz. Límite Sur; Municipios Coro Coro, Calacoto y Charaña que son Primera, Tercera y Quinta Sección de la provincia Pacajes del departamento de La Paz. Límite Este; Municipio Comanche que es Cuarta Sección de la provincia Pacajes del departamento de La Paz. Límite Oeste; Municipio de Santiago de Machaca de la provincia José Manuel Pando del departamento de La Paz. 1.1.3. Extensión La Provincia Pacajes posee una extensión total de 10.584 km², de la cual la Segunda Sección Municipal Caquiaviri representa el 14 % del total de la provincia, lo que equivale a una superficie de 1.478 Km². -

Caryophyllaceae)
4 LUNDELLIA DECEMBER, 2017 NOMENCLATURAL NOTES ON THE ANDEAN GENERA PYCNOPHYLLOPSIS AND PYCNOPHYLLUM (CARYOPHYLLACEAE) Martın´ E. Timana´ Departamento de Humanidades, Seccion´ Geografıa,´ and Centro de Investigacion´ en Geografıa´ Aplicada (CIGA) Pontificia Universidad Catolica´ del Peru,´ Av. Universitaria 1801, San Miguel, Lima 32. Peru´ Email: [email protected] Abstract: The nomenclature of the high Andean genera Pycnophyllopsis Skottsb. and Pycnophyllum J. Remy´ is examined. Eight species of Pycnophyllopsis are recognized; lectotypes or neotypes are selected when required; a new species, Pycnophyllopsis smithii is proposed and two new combinations are made. The genus Plettkea Mattf. is reduced to a synonym of Pycnophyllopsis. Ten species of Pycnophyllum are accepted, including a new species, Pycnophyllum huascaranum and lectotypes or neotypes are selected when needed. Resumen: Se examina la nomenclatura de los generos´ altoandinos Pycnophyllopsis Skottsb. y Pycnophyllum J. Remy.´ Se reconocen ocho especies de Pycnophyllopsis; se designan lectotipos y neotipos cuando es requerido; se propone una nueva especie, Pycnophyllopsis smithii, y dos nuevas combinaciones. Se aceptan diez especies de Pycnophyllum, incluyendo una nueva especie, Pycnophyllum huascaranum; se designan lectotipos y neotipos cuando es requerido. Keywords: Caryophyllaceae, Alsinoideae, Pycnophyllopsis, Pycnophyllum, Plettkea, Andes, nomenclature, Peru, Bolivia. The Caryophyllaceae consists of 100 Andes and the mountain regions of North genera and almost 3000 species (Herna´ndez and Central America. Some genera (includ- et al., 2015). Traditionally, the family has ing several endemics) of the Caryophyllaceae been divided into three subfamilies (Alsi- reach the southern hemisphere, particularly noideae Fenzl, Caryophylloideae Arnott , the high Andes and the south temperate and and Paronychioideae Meisner, but see Har- sub-Antarctic regions. -

Catastro Arqueológico Del Municipio Jesús De Machaca
Catastro Arqueológico del Municipio Jesús de Machaca Catastro Arqueológico del Municipio Jesús de Machaca Coordinadores Jimena Portugal Loayza Huber Catacora Alvarado Investigadores Salvador Arano Romero José Miguel Velasco Mamani Colaboradores Wendy Chávez Irusta Gabriel Morales Medrano Ángel Becerra Krings © Universidad Mayor de San Andrés, 2017 Primera edición: diciembre de 2017 D.L.: 4-1-560-17 P.O. ISBN: 978-99974-981-0-6 Producción Plural editores Av. Ecuador 2337 esq. calle Rosendo Gutiérrez Teléfono: 2411018 / Casilla Postal 5097, La Paz, Bolivia e-mail: [email protected] / www.plural.bo Impreso en Bolivia Índice Presentación Serie Catastros Arqueológicos ..................................................................................... 11 CAPÍTULO I El Catastro Arqueológico como parte de la Cartografía Cultural Jimena Portugal Loayza ................................................................................................. 15 CAPÍTULO II El área del estudio y antecedentes de la investigación Juan Gabriel Morales Medrano Wendy Rocío Chávez Irusta Salvador Arano Romero José Miguel Velasco Mamani ........................................................................................... 27 CAPÍTULO III Viviendo entre Chullpas, socavones y Markas ancestrales. Percepciones de los pobladores de Jesús de Machaca acerca de los sitios arqueológicos José Miguel Velasco Mamani ........................................................................................... 37 CAPÍTULO IV Sitios registrados en el Catastro Arqueológico -

PNAAH514.Pdf
BIBLIOGRAPIC DATA SHEET ONROLNNUMBER 2. SUBJECT CLASSIFICATION (695) PN-AAH- 514 NAOO-0000-G512 3. TITLE AND SUBTITLE (240) Bolivia health sector asses;ment 4. PERSONAL AUTHORS (100) %andry, A. So 5. C'ORPORATE AUTHORS (101) AID/LA/U SAID/Bolivia 6. DOCUMENT DATE (110) 17. NUMBER OF PAGES (120) 8. ARC NUMBFhR (170) 1975 512p. I B.bI4.0984.L262 9. REFERENCE ORGANIZATION (130) Bolivia 10. SUPPLEMENTARY NOTES (500) 1I. ABSTRACT (950) i.DESu'-Rs (92o) 'US. FrOjECr Uviu a (N5UB Sector analysis Infrastructure Bolivia Health Public health Socioezonomic deve].opment 14. CONTRACT NO.(140) 15. CONTRACT Program planning Health services TYPE (140) Socioeconomic status Bolivia 16. TPE oF DOCUMENT (160) 16 AM 5Wo7 (10-79) LAC/DR/RD's copy - please return LA/DR-DAEC/P-7q- 50 Bolivia Health SectorAssessment '9 . n 5._._.._ .46. e.• • .. ., qr" -. K >'.;._, ,. : .. ,. , ,- .. ." . .. .. ... ,, . "" . " - . "' : - * -*.. I . • • qm~i ,. United States AID Mission to BolivaS ..... ... W... .. .... .. .. .... .. ..Janua r y.1 97/5 . ... .... ... .". BOLIVIA HEALTH SECTOR ASSESSMENT .UNITED STATES AID MISSION TO BOLIVIA L%PAZ, JANUARY 1975 PREFACE The ultimate objective of the U.S. technical and financial ance programs assist is to help the Republic of Bolivia attain its socio-eco nomic development goals. The following Health Sector Assessment one of three* is efforts desig. d to analyze the current status of sector activities in Bolivia and to pcovide guidance for planning future USAID programs. It is the intent of the Mission to provide coordinated, mutually supportive interventions in the agriculture, education and health sectors. A simultaneous review of health problems and programs taken by a specially was under convened Interministerial Commission. -

Geography of the Central Andes
: ' ; ,!.',:.,': 1 : -.. :;i:. / - ..; ^i;.^!. i;J ;.;.;.;> ; ,\v 'J.'' GEOGRAPHY OF THE ALAN G. OGILVIE AMERICAN GEOGRAPHICAL,. SOCIETY map of Hispanic America- PUBLrcAT.iON#o, t . Digitized by the Internet Archive in 2011 with funding from The Library of Congress http://www.archive.org/details/geographyofcentrOOogil -HV OF THE ) AMERICAN GEOGRAPHICAL SOCIETY MAP OF HISPANIC AMERICA PUBLICATION NO. i , GEOGRAPHY OF THE CENTRAL ANDES A Handbook to Accompany the LA PAZ Sheet of the Map of Hispanic America on the Millionth Scale BY ALAN G. OGILVIE, M.A., B.Sc. (Oxon.) WITH AN INTRODUCTION BY ISAIAH BOWMAN PUBLISHED BY THE AMERICAN GEOGRAPHICAL SOCIETY OF NEW YORK BROADWAY AT I56TH STREET 1922 Vzziz COPYRIGHT, 1922 BY THE AMERICAN GEOGRAPHICAL SOCIETY OF NEW YORK THE CONDE NAST PRESS GREENWICH, CONN. N-8'23 ©CH690901 CONTENTS PART I THE LA PAZ SHEET OF THE 1:1,000,000 MAP PART II GEOGRAPHY OF THE LA PAZ SHEET AREA CHAPTER PAGE I General View 13 II Geological Structure and Land Forms .... 31 III Minerals and Mines 49 IV The Ocean \ . 61 V The Climate 67 VI Drainage, Water Supply, and Soils 95 VII The Natural Vegetation 109 VIII Animal Life 122 IX The Inhabitants and Their Adaptation to the Environment 136 Appendix A, The Social and Religious Organiza- tion of the Plateau Indians ... 199 Appendix B, The Problem of Tacna and Arica . 203 Appendix C, Selected Bibliography 211 Appendix D, Conversion Tables 224 Index 233 1 1 LIST OF ILLUSTRATIONS PLATE PAGE I Map showing distribution of population .... facing 146 II Map showing utilization of land facing 174 FIG. -

New Data on Tectonics of Bolivian Andes from a Photograph by Gemini 5, and Field Knowledges
[Trav. Lab. Gébl. Grenoble, t. 42, 1966] New data on Tectonics of Bolivian Andes from a photograph by Gemini 5, and field knowledges by Luigi RADELLI To my children, who will fly among the planets. ABSTRACT : A photograph taken by Gemini 5 shows many of the basic géomorphologie units of Bolivian Andes, which are : the Western volcanic Cordiller.a; the Altiplano with the lakes of Titikaka and Poopo, and <the Sait lakes of Uyuni and Coipasa; the Huayllamarca Range; the Eastern Cordilleras, including Cordillera Real; and the Sub Andean Zone. The whole of Bolivian Andes is eut by several tectonic lines, either paraHel or transverse to the géographie trend of the mountain ranges, some ones of which were revealed first by the photograph taken from Gemini 5. The tectonic lines (Coniri, Ichilo-Achacachi, Poopo, Palca, Chacarilla, and Chiriquina) correspond to faults which were active ever since Paleozoic. Their existence is proved by précise both stratigraphie and tectonic remarks within the blocks separated by them- selves. Of those, the Poopo Line crosses the Andean géographie trend; the Ichilo-Achacachi one does also that between the town of Santa Gruz on the East and the Cordillera Real on the West, but then it becomes paraUel to this trend. The other ones are always parallel to it. Mutual movements of Andean blocks separated by the indicated intersecting faults occurred during géologie time are studied since Silurian, and they are summarized at S 18. Spécial attention is paid to both Hercynian and Nevadian tectonic movements. With regard to Bolivian Andes, the conclusion is attained that one Hercynian cordillera existed Eastwards of the actual Altiplano, ano'ther Westwards of it, and a minor third one in place of Altiplano itself, Eastwards of tihe actual Huayllamarca Range. -
Copyright by Martin Enrique Timaná 2005
Copyright by Martin Enrique Timaná 2005 The Dissertation Committee for Martin Enrique Timaná Certifies that this is the approved version of the following dissertation: SYSTEMATIC STUDIES IN PYCNOPHYLLUM AND PYCNOPHYLLOPSIS (CARYOPHYLLACEAE) OF THE HIGH ANDES Committee: ______________________________ Beryl B. Simpson, Supervisor ______________________________ David Cannatella ______________________________ Robert K. Jansen ______________________________ Tom J. Mabry ______________________________ Jose L. Panero SYSTEMATIC STUDIES IN PYCNOPHYLLUM AND PYCNOPHYLLOPSIS (CARYOPHYLLACEAE) OF THE HIGH ANDES by Martin Enrique Timaná, B.Sc.; M.Sc. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin May, 2005 Dedication To my son, Leonardo Martin, miracle child and first Texan in the family, For offering me a smile every single day since birth, And to my wife, Aleyda My friend and companion for many years, For waiting this long It was the best of times, it was the worst of times… Charles Dickens, A Tale of Two Cities For sudden, the worst turns the best to the brave… Robert Browing, Prospice Acknowledgements This Dissertation represents the work of several years as a graduate student at the University of Texas. I would like to thank first and above all my graduate advisor, Dr. Beryl B. Simpson, for her infinite patience and support and the intellectual freedom I received from her during all these years. Thank you, Beryl, with all my heart for believing in me. Based on reading my fellow graduate students’ dissertations, it seems to me that this Acknowledgements section is probably the only segment where (unless I’m told otherwise) I have almost all the freedom to express my personal thoughts in my own style, without either being subject to the approval (or disapproval) of members of my dissertation committee or constrained by the stiff and unattractive style of academic prose.