S.I. and Cgs Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modern Physics Unit 15: Nuclear Structure and Decay Lecture 15.1: Nuclear Characteristics
Modern Physics Unit 15: Nuclear Structure and Decay Lecture 15.1: Nuclear Characteristics Ron Reifenberger Professor of Physics Purdue University 1 Nucleons - the basic building blocks of the nucleus Also written as: 7 3 Li ~10-15 m Examples: A X=Chemical Element Z X Z = number of protons = atomic number 12 A = atomic mass number = Z+N 6 C N= A-Z = number of neutrons 35 17 Cl 2 What is the size of a nucleus? Three possibilities • Range of nuclear force? • Mass radius? • Charge radius? It turns out that for nuclear matter Nuclear force radius ≈ mass radius ≈ charge radius defines nuclear force range defines nuclear surface 3 Nuclear Charge Density The size of the lighter nuclei can be approximated by modeling the nuclear charge density ρ (C/m3): t ≈ 4.4a; a=0.54 fm 90% 10% Usually infer the best values for ρo, R and a for a given nucleus from scattering experiments 4 Nuclear mass density Scattering experiments indicate the nucleus is roughly spherical with a radius given by 1 3 −15 R = RRooA ; =1.07 × 10meters= 1.07 fm = 1.07 fermis A=atomic mass number What is the nuclear mass density of the most common isotope of iron? 56 26 Fe⇒= A56; Z = 26, N = 30 m Am⋅⋅3 Am 33A ⋅ m m ρ = nuc p= p= pp = o 1 33 VR4 3 3 3 44ππA R nuc π R 4(π RAo ) o o 3 3×× 1.66 10−27 kg = 3.2× 1017kg / m 3 4×× 3.14 (1.07 × 10−15m ) 3 The mass density is constant, independent of A! 5 Nuclear mass density for 27Al, 97Mo, 238U (from scattering experiments) (kg/m3) ρo heavy mass nucleus light mass nucleus middle mass nucleus 6 Typical Densities Material Density Helium 0.18 kg/m3 Air (dry) 1.2 kg/m3 Styrofoam ~100 kg/m3 Water 1000 kg/m3 Iron 7870 kg/m3 Lead 11,340 kg/m3 17 3 Nuclear Matter ~10 kg/m 7 Isotopes - same chemical element but different mass (J.J. -

Units and Magnitudes (Lecture Notes)
physics 8.701 topic 2 Frank Wilczek Units and Magnitudes (lecture notes) This lecture has two parts. The first part is mainly a practical guide to the measurement units that dominate the particle physics literature, and culture. The second part is a quasi-philosophical discussion of deep issues around unit systems, including a comparison of atomic, particle ("strong") and Planck units. For a more extended, profound treatment of the second part issues, see arxiv.org/pdf/0708.4361v1.pdf . Because special relativity and quantum mechanics permeate modern particle physics, it is useful to employ units so that c = ħ = 1. In other words, we report velocities as multiples the speed of light c, and actions (or equivalently angular momenta) as multiples of the rationalized Planck's constant ħ, which is the original Planck constant h divided by 2π. 27 August 2013 physics 8.701 topic 2 Frank Wilczek In classical physics one usually keeps separate units for mass, length and time. I invite you to think about why! (I'll give you my take on it later.) To bring out the "dimensional" features of particle physics units without excess baggage, it is helpful to keep track of powers of mass M, length L, and time T without regard to magnitudes, in the form When these are both set equal to 1, the M, L, T system collapses to just one independent dimension. So we can - and usually do - consider everything as having the units of some power of mass. Thus for energy we have while for momentum 27 August 2013 physics 8.701 topic 2 Frank Wilczek and for length so that energy and momentum have the units of mass, while length has the units of inverse mass. -

Measuring Vibration Using Sound Level Meter Nor140
Application Note Measuring vibration using sound level meter Nor140 This technical note describes the use of the sound level meter Nor140 for measurement of acceleration levels. The normal microphone is then substituted by an accelerometer. The note also describes calibration and the relation between the level in decibel and the acceleration in linear units. AN Vibration Ed2Rev0 English 03.08 AN Vibration Ed2Rev0 English 03.08 Introduction Accelerometer Most sound level meters and sound analysers can be used for Many types of acceleration sensitive sensors exist. For vibration measurements, even if they do not provide absolute connection to Nor140 sound level meter the easiest is to (linear) units in the display. To simplify the description, this ap- apply an ICP® or CCP type. This type of transducers has low plication note describes the use of the handheld sound level output impedance and may be supplied through a coaxial meter Norsonic Nor140. However, the described principles able. Nor1270 (Sens. 10 mV/ms-2; 23 g) and Nor1271 (Sens. also apply to other types of sound level meters. 1,0 mV/ms-2; 3,5 g) are recommended. Connect the accelero- Although several transducer principles are commercially meter through the BNC/Lemo cable Nor1438 and the BNC to available, this application note will deal with the accelerometer microdot adaptor Nor1466. You also need a BNC-BNC female only, simply because it is the transducer type most commonly connector. See the figure below. encountered when measuring vibration levels. As the name Alternatively, a charge sensitive accelerometer may be suggests, the accelerometer measures the acceleration it is used and coupled to the normal microphone preamplifier exposed to and provides an output signal proportional to the Nor1209 through the adapter with BNC input Nor1447/2 and instant acceleration. -

Coulomb's Force
COULOMB’S FORCE LAW Two point charges Multiple point charges Attractive Repulsive - - + + + The force exerted by one point charge on another acts along the line joining the charges. It varies inversely as the square of the distance separating the charges and is proportional to the product of the charges. The force is repulsive if the charges have the same sign and attractive if the charges have opposite signs. q1 Two point charges q1 and q2 q2 [F]-force; Newtons {N} [q]-charge; Coulomb {C} origin [r]-distance; meters {m} [ε]-permittivity; Farad/meter {F/m} Property of the medium COULOMB FORCE UNIT VECTOR Permittivity is a property of the medium. Also known as the dielectric constant. Permittivity of free space Coulomb’s constant Permittivity of a medium Relative permittivity For air FORCE IN MEDIUM SMALLER THAN FORCE IN VACUUM Insert oil drop Viewing microscope Eye Metal plates Millikan oil drop experiment Charging by contact Example (Question): A negative point charge of 1µC is situated in air at the origin of a rectangular coordinate system. A second negative point charge of 100µC is situated on the positive x axis at the distance of 500 mm from the origin. What is the force on the second charge? Example (Solution): A negative point charge of 1µC is situated in air at the origin of a rectangular coordinate system. A second negative point charge of 100µC is situated on the positive x axis at the distance of 500 mm from the origin. What is the force on the second charge? Y q1 = -1 µC origin X q2= -100 µC Example (Solution): Y q1 = -1 µC origin X q2= -100 µC END Multiple point charges It has been confirmed experimentally that when several charges are present, each exerts a force given by on every other charge. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Gravity and Coulomb's
Gravity operates by the inverse square law (source Hyperphysics) A main objective in this lesson is that you understand the basic notion of “inverse square” relationships. There are a small number (perhaps less than 25) general paradigms of nature that if you make them part of your basic view of nature they will help you greatly in your understanding of how nature operates. Gravity is the weakest of the four fundamental forces, yet it is the dominant force in the universe for shaping the large-scale structure of galaxies, stars, etc. The gravitational force between two masses m1 and m2 is given by the relationship: This is often called the "universal law of gravitation" and G the universal gravitation constant. It is an example of an inverse square law force. The force is always attractive and acts along the line joining the centers of mass of the two masses. The forces on the two masses are equal in size but opposite in direction, obeying Newton's third law. You should notice that the universal gravitational constant is REALLY small so gravity is considered a very weak force. The gravity force has the same form as Coulomb's law for the forces between electric charges, i.e., it is an inverse square law force which depends upon the product of the two interacting sources. This led Einstein to start with the electromagnetic force and gravity as the first attempt to demonstrate the unification of the fundamental forces. It turns out that this was the wrong place to start, and that gravity will be the last of the forces to unify with the other three forces. -

James Clerk Maxwell
Conteúdo Páginas Henry Cavendish 1 Jacques Charles 2 James Clerk Maxwell 3 James Prescott Joule 5 James Watt 7 Jean-Léonard-Marie Poiseuille 8 Johann Heinrich Lambert 10 Johann Jakob Balmer 12 Johannes Robert Rydberg 13 John Strutt 14 Michael Faraday 16 Referências Fontes e Editores da Página 18 Fontes, Licenças e Editores da Imagem 19 Licenças das páginas Licença 20 Henry Cavendish 1 Henry Cavendish Referência : Ribeiro, D. (2014), WikiCiências, 5(09):0811 Autor: Daniel Ribeiro Editor: Eduardo Lage Henry Cavendish (1731 – 1810) foi um físico e químico que investigou isoladamente de acordo com a tradição de Sir Isaac Newton. Cavendish entrou no seminário em 1742 e frequentou a Universidade de Cambridge (1749 – 1753) sem se graduar em nenhum curso. Mesmo antes de ter herdado uma fortuna, a maior parte das suas despesas eram gastas em montagem experimental e livros. Em 1760, foi eleito Fellow da Royal Society e, em 1803, um dos dezoito associados estrangeiros do Institut de France. Entre outras investigações e descobertas de Cavendish, a maior ocorreu em 1781, quando compreendeu que a água é uma substância composta por hidrogénio e oxigénio, uma reformulação da opinião de há milénios de que a água é um elemento químico básico. O químico inglês John Warltire (1725/6 – 1810) realizou uma Figura 1 Henry Cavendish (1731 – 1810). experiência em que explodiu uma mistura de ar e hidrogénio, descobrindo que a massa dos gases residuais era menor do que a da mistura original. Ele atribuiu a perda de massa ao calor emitido na reação. Cavendish concluiu que algum erro substancial estava envolvido visto que não acreditava que, dentro da teoria do calórico, o calor tivesse massa suficiente, à escala em análise. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -
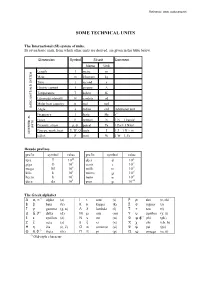
Technical Units
Reference: www. rockmass.net 620(7(&+1,&$/81,76 7KH,QWHUQDWLRQDO 6, V\VWHPRIXQLWV Its seven basic units, from which other units are derived, are given in the table below. Dimension Symbol SI unit Comment Name Unit Length l metre m Mass m kilogram kg Time t second s Electric current I ampere A Temperature T kelvin K Luminous intensity Iv candela cd Molar heat capacity n mol mol BASIC UNITS IN SI SYSTEM Angle a radian rad Additional unit Frequency f hertz Hz s-1 Force F newton N 1 N = 1 kgm/s2 Pressure, stress p, σ, pascal Pa 1 Pa = 1 N/m² UNITS Energy, work, heat E, W, Q joule J 1 J = 1 N × m DIVERTED SI- Effect P watt W 1 W = 1 J/s 'HFDGHSUHIL[HV prefix symbol value prefix symbol value tera T 1012 deci d 10-1 giga G 109 centi c 10-2 mega M 106 milli m 10-3 kilo k 103 micro µ 10-6 hecto h 102 nano n 10-9 deca da 101 pico p 10-12 7KH*UHHNDOSKDEHW Α α, ∝ ∗) alpha (a) Ι ι iota (i) Ρ ρ rho (r, rh) Β β beta (b) Κ κ kappa (k) Σ σ sigma (s) Γ γ gamma (g, n) Λ λ lambda (l) Τ τ tau (t) ∆ δ, ∂ ∗) delta (d) Μ µ mu (m) Υ υ upsilon (y, u) Ε ε epsilon (e) Ν ν nu (n) Φ φ, ϕ*) phi (ph) Ζ ζ zeta (z) Ξ ξ xi (x) X χ chi (ch, h) Η η ëta (e, ë) Ο ο omicron (o) Ψ ψ psi (ps) Θ θ, ϑ ∗) theta (th) Π π pi (p) Ω ω omega (o, õ) *) Old-style character 2 9DULRXVXQLWVDQGFRQYHUVLRQV 'LPHQVLRQ 1DPH 6\PERO 8QLW &RQYHUVLRQ Time minute min s 1 h = 60 s hour h h 1 h = 60 min day day day 1 day = 24 h week wk wk 1 wk = 7 days year yr yr 1 yr = 365.242 days = 52.17 wk Length Angstrom A m 1 A = 10-10 m nautical mile n mile 1 n mile = 1852 m wave length λ m Force load G (P,W) N 1 kp = 9.807 N 2 o o 2 gravity g N 1 gn = 9.80665 m/s (exact, at 45 N; at 60 N g = 9.82 m/s ) atmosphere atm 1 atm Modulus elasticity modulus E Pa 1 kPa = 0.1 N/cm = 0.102 ton/m2 deformation modulus G Pa 1 MPa = 10,2 kg/cm2 shear modulus K Pa Density mass density ρ kg/m3 force density γ kN/m3 Flow Lugeon Lugeon l/min/m 1 Lugeon = approx. -

DIMENSIONS and UNITS to Get the Value of a Quantity in Gaussian Units, Multiply the Value Ex- Pressed in SI Units by the Conversion Factor
DIMENSIONS AND UNITS To get the value of a quantity in Gaussian units, multiply the value ex- pressed in SI units by the conversion factor. Multiples of 3 intheconversion factors result from approximating the speed of light c =2.9979 1010 cm/sec × 3 1010 cm/sec. ≈ × Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units t2q2 Capacitance C l farad 9 1011 cm ml2 × m1/2l3/2 Charge q q coulomb 3 109 statcoulomb t × q m1/2 Charge ρ coulomb 3 103 statcoulomb 3 3/2 density l l t /m3 × /cm3 tq2 l Conductance siemens 9 1011 cm/sec ml2 t × 2 tq 1 9 1 Conductivity σ siemens 9 10 sec− 3 ml t /m × q m1/2l3/2 Current I,i ampere 3 109 statampere t t2 × q m1/2 Current J, j ampere 3 105 statampere 2 1/2 2 density l t l t /m2 × /cm2 m m 3 3 3 Density ρ kg/m 10− g/cm l3 l3 q m1/2 Displacement D coulomb 12π 105 statcoulomb l2 l1/2t /m2 × /cm2 1/2 ml m 1 4 Electric field E volt/m 10− statvolt/cm t2q l1/2t 3 × 2 1/2 1/2 ml m l 1 2 Electro- , volt 10− statvolt 2 motance EmfE t q t 3 × ml2 ml2 Energy U, W joule 107 erg t2 t2 m m Energy w, ϵ joule/m3 10 erg/cm3 2 2 density lt lt 10 Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units ml ml Force F newton 105 dyne t2 t2 1 1 Frequency f, ν hertz 1 hertz t t 2 ml t 1 11 Impedance Z ohm 10− sec/cm tq2 l 9 × 2 2 ml t 1 11 2 Inductance L henry 10− sec /cm q2 l 9 × Length l l l meter (m) 102 centimeter (cm) 1/2 q m 3 Magnetic H ampere– 4π 10− oersted 1/2 intensity lt l t turn/m × ml2 m1/2l3/2 Magnetic flux Φ weber 108 maxwell tq t m m1/2 Magnetic -

Communications-Mathematics and Applied Mathematics/Download/8110
A Mathematician's Journey to the Edge of the Universe "The only true wisdom is in knowing you know nothing." ― Socrates Manjunath.R #16/1, 8th Main Road, Shivanagar, Rajajinagar, Bangalore560010, Karnataka, India *Corresponding Author Email: [email protected] *Website: http://www.myw3schools.com/ A Mathematician's Journey to the Edge of the Universe What’s the Ultimate Question? Since the dawn of the history of science from Copernicus (who took the details of Ptolemy, and found a way to look at the same construction from a slightly different perspective and discover that the Earth is not the center of the universe) and Galileo to the present, we (a hoard of talking monkeys who's consciousness is from a collection of connected neurons − hammering away on typewriters and by pure chance eventually ranging the values for the (fundamental) numbers that would allow the development of any form of intelligent life) have gazed at the stars and attempted to chart the heavens and still discovering the fundamental laws of nature often get asked: What is Dark Matter? ... What is Dark Energy? ... What Came Before the Big Bang? ... What's Inside a Black Hole? ... Will the universe continue expanding? Will it just stop or even begin to contract? Are We Alone? Beginning at Stonehenge and ending with the current crisis in String Theory, the story of this eternal question to uncover the mysteries of the universe describes a narrative that includes some of the greatest discoveries of all time and leading personalities, including Aristotle, Johannes Kepler, and Isaac Newton, and the rise to the modern era of Einstein, Eddington, and Hawking. -

CAR-ANS Part 5 Governing Units of Measurement to Be Used in Air and Ground Operations
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila UNCOTROLLED COPY INTENTIONALLY LEFT BLANK UNCOTROLLED COPY CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 UNCOTROLLED COPY CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned.