Technical Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

James Clerk Maxwell
Conteúdo Páginas Henry Cavendish 1 Jacques Charles 2 James Clerk Maxwell 3 James Prescott Joule 5 James Watt 7 Jean-Léonard-Marie Poiseuille 8 Johann Heinrich Lambert 10 Johann Jakob Balmer 12 Johannes Robert Rydberg 13 John Strutt 14 Michael Faraday 16 Referências Fontes e Editores da Página 18 Fontes, Licenças e Editores da Imagem 19 Licenças das páginas Licença 20 Henry Cavendish 1 Henry Cavendish Referência : Ribeiro, D. (2014), WikiCiências, 5(09):0811 Autor: Daniel Ribeiro Editor: Eduardo Lage Henry Cavendish (1731 – 1810) foi um físico e químico que investigou isoladamente de acordo com a tradição de Sir Isaac Newton. Cavendish entrou no seminário em 1742 e frequentou a Universidade de Cambridge (1749 – 1753) sem se graduar em nenhum curso. Mesmo antes de ter herdado uma fortuna, a maior parte das suas despesas eram gastas em montagem experimental e livros. Em 1760, foi eleito Fellow da Royal Society e, em 1803, um dos dezoito associados estrangeiros do Institut de France. Entre outras investigações e descobertas de Cavendish, a maior ocorreu em 1781, quando compreendeu que a água é uma substância composta por hidrogénio e oxigénio, uma reformulação da opinião de há milénios de que a água é um elemento químico básico. O químico inglês John Warltire (1725/6 – 1810) realizou uma Figura 1 Henry Cavendish (1731 – 1810). experiência em que explodiu uma mistura de ar e hidrogénio, descobrindo que a massa dos gases residuais era menor do que a da mistura original. Ele atribuiu a perda de massa ao calor emitido na reação. Cavendish concluiu que algum erro substancial estava envolvido visto que não acreditava que, dentro da teoria do calórico, o calor tivesse massa suficiente, à escala em análise. -

S.I. and Cgs Units
Some Notes on SI vs. cgs Units by Jason Harlow Last updated Feb. 8, 2011 by Jason Harlow. Introduction Within “The Metric System”, there are actually two separate self-consistent systems. One is the Systme International or SI system, which uses Metres, Kilograms and Seconds for length, mass and time. For this reason it is sometimes called the MKS system. The other system uses centimetres, grams and seconds for length, mass and time. It is most often called the cgs system, and sometimes it is called the Gaussian system or the electrostatic system. Each system has its own set of derived units for force, energy, electric current, etc. Surprisingly, there are important differences in the basic equations of electrodynamics depending on which system you are using! Important textbooks such as Classical Electrodynamics 3e by J.D. Jackson ©1998 by Wiley and Classical Electrodynamics 2e by Hans C. Ohanian ©2006 by Jones & Bartlett use the cgs system in all their presentation and derivations of basic electric and magnetic equations. I think many theorists prefer this system because the equations look “cleaner”. Introduction to Electrodynamics 3e by David J. Griffiths ©1999 by Benjamin Cummings uses the SI system. Here are some examples of units you may encounter, the relevant facts about them, and how they relate to the SI and cgs systems: Force The SI unit for force comes from Newton’s 2nd law, F = ma, and is the Newton or N. 1 N = 1 kg·m/s2. The cgs unit for force comes from the same equation and is called the dyne, or dyn. -

DIMENSIONS and UNITS to Get the Value of a Quantity in Gaussian Units, Multiply the Value Ex- Pressed in SI Units by the Conversion Factor
DIMENSIONS AND UNITS To get the value of a quantity in Gaussian units, multiply the value ex- pressed in SI units by the conversion factor. Multiples of 3 intheconversion factors result from approximating the speed of light c =2.9979 1010 cm/sec × 3 1010 cm/sec. ≈ × Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units t2q2 Capacitance C l farad 9 1011 cm ml2 × m1/2l3/2 Charge q q coulomb 3 109 statcoulomb t × q m1/2 Charge ρ coulomb 3 103 statcoulomb 3 3/2 density l l t /m3 × /cm3 tq2 l Conductance siemens 9 1011 cm/sec ml2 t × 2 tq 1 9 1 Conductivity σ siemens 9 10 sec− 3 ml t /m × q m1/2l3/2 Current I,i ampere 3 109 statampere t t2 × q m1/2 Current J, j ampere 3 105 statampere 2 1/2 2 density l t l t /m2 × /cm2 m m 3 3 3 Density ρ kg/m 10− g/cm l3 l3 q m1/2 Displacement D coulomb 12π 105 statcoulomb l2 l1/2t /m2 × /cm2 1/2 ml m 1 4 Electric field E volt/m 10− statvolt/cm t2q l1/2t 3 × 2 1/2 1/2 ml m l 1 2 Electro- , volt 10− statvolt 2 motance EmfE t q t 3 × ml2 ml2 Energy U, W joule 107 erg t2 t2 m m Energy w, ϵ joule/m3 10 erg/cm3 2 2 density lt lt 10 Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units ml ml Force F newton 105 dyne t2 t2 1 1 Frequency f, ν hertz 1 hertz t t 2 ml t 1 11 Impedance Z ohm 10− sec/cm tq2 l 9 × 2 2 ml t 1 11 2 Inductance L henry 10− sec /cm q2 l 9 × Length l l l meter (m) 102 centimeter (cm) 1/2 q m 3 Magnetic H ampere– 4π 10− oersted 1/2 intensity lt l t turn/m × ml2 m1/2l3/2 Magnetic flux Φ weber 108 maxwell tq t m m1/2 Magnetic -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Appendix 9 Updated MIG 0 15.3
Units of Measure [UNECE Recommendation No. 20] Code Description 05 lift 06 small spray 08 heat lot 10 group 11 outfit 13 ration 14 shot 15 stick, military 16 hundred fifteen kg drum 17 hundred lb drum 18 fiftyfive gallon (US) drum 19 tank truck 20 twenty foot container 21 forty foot container 22 decilitre per gram 23 gram per cubic centimetre 24 theoretical pound 25 gram per square centimetre 26 actual ton 27 theoretical ton 28 kilogram per square metre 29 pound per thousand square foot 30 horse power day per air dry metric ton 31 catch weight 32 kilogram per air dry metric ton 33 kilopascal square metre per gram 34 kilopascal per millimetre 35 millilitre per square centimetre second 36 cubic foot per minute per square foot 37 ounce per square foot 38 ounce per square foot per 0,01inch 40 millilitre per second 41 millilitre per minute 43 super bulk bag 44 fivehundred kg bulk bag 45 threehundred kg bulk bag 46 fifty lb bulk bag 47 fifty lb bag 48 bulk car load 53 theoretical kilogram 54 theoretical tonne 56 sitas 57 mesh 58 net kilogram 59 part per million 60 percent weight 61 part per billion (US) 62 percent per 1000 hour 63 failure rate in time 64 pound per square inch, gauge 66 oersted 69 test specific scale 71 volt ampere per pound 72 watt per pound 73 ampere tum per centimetre 74 millipascal 76 gauss 77 milli-inch 78 kilogauss 80 pound per square inch absolute 81 henry 84 kilopound-force per square inch 85 foot pound-force 87 pound per cubic foot 89 poise 90 Saybold universal second 91 stokes 92 calorie per cubic centimetre 93 calorie -

Aldrich Polymer Products Application & Reference Information
Reference:Reference: PolymerPolymer PropertiesProperties Viscosity Viscosity Terms η rel is determined by the ratio t/t0, where (t) is the outflow time of a given volume of solution and 0(t) is the outflow time of the equivalent volume of pure solvent. Values are commonly determined with a capillary viscometer. Other viscosity terms are defined in Table I. Table I: Definition of Viscosity Terms IUPAC Name Common Name Definition Symbol Units η η η Viscosity ratio Relative viscosity / 0 rel dimensionless η/η η Specific viscosity Specific viscosity 0 -1 sp dimensionless η η Viscosity number Reduced viscosity sp /c red deciliter/gram η η Logarithmic viscosity number Inherent viscosity In rel /c inh deciliter/gram Limiting viscosity number Intrinsic viscosity lim (η /c) [η] deciliter/gram c ← 0 sp lim (ln η /c) c ← 0 rel Absolute viscosity P Poise (dynes · s/cm) Pa · s Pascal · second (Newton · s/m2) Kinematic Viscosity St Stokes (m2/s) Conversion factor: η(Poise) = η(Stoke) x density (g/cm3) Viscometer Comparison for NEWTONIAN Liquids The chart1 below is intended to be an aid in comparing viscometer measurements of Newtonian liquids by referencing to absolute and kinematic viscosity. Centistokes Reference Mobilometer 100g 10cmsec. Engler degrees Ford 4 sec. Ford 3 sec. Saybolt Universal sec. Saybolt Furol sec. Redwood Standard "1 sec. Ubbelohde cks. Gardner Holts cks. Zahn 5 sec. Zahn 3 sec. Kreb Stormer 200G K.U. Stormer Cyl. 150G sec. Brookfield Cps. Centipoise Reference 1 Reprinted with permission from Brookfield Engineer ing Laboratories, Inc., Stoughton, MA, U.S.A. 50 Polymer Products from Aldrich a Reference:Reference: PolymerPolymer PropertiesProperties Viscosity (continued) Viscosity - Molecular Weight Relationships Mark-Houwink-Sakurada Equation: a [η]=KM...................... -

Massachusetts Arts Curriculum Framework
Massachusetts Arts Curriculum Framework November 1999 Massachusetts Department of Elementary & Secondary Education 350 Main Street, Malden, MA 02148 Phone 781-338-3000 TTY: N.E.T. Relay 800-439-2370 www.doe.mass.edu Massachusetts Arts Curriculum Framework October 1999 October, 1999 Dear Colleagues, I am pleased to present to you the Massachusetts Arts Curriculum Framework that was adopted by the Board of Education in June, 1999. This second edition of the Arts Curriculum Framework presents the new statewide guidelines for learning, teaching, and assessment in dance, music, theatre, and visual arts for the Commonwealth’s public schools. Based on scholarship, sound research, and effective practice, the Framework will enable teachers and administrators to strengthen curriculum and instruction from PreKindergarten through grade 12. I am proud of the work that has been accomplished. The comments and suggestions received on the first edition of the Arts Curriculum Framework of 1996, as well as comments on subsequent working drafts, have strengthened this new edition. I want to thank everyone who worked with us to create a high quality document that provides challenging learning standards for Massachusetts students. We will continue to work with schools and districts in implementing the Arts Curriculum Framework over the next several years, and we encourage your comments as you use it. All of the curriculum frameworks are subject to continuous review and improvement, for the benefit of the students of the Commonwealth. Thank you again for your -
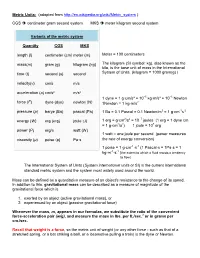
Metric Units.Pdf
Metric Units: (adapted from http://en.wikipedia.org/wiki/Metric_system ) CGS centimeter gram second system MKS meter kilogram second system Variants of the metric system Quantity CGS MKS length (l) centimeter (cm) meter (m) Meter = 100 centimeters mass(m) gram (g) kilogram (kg) The kilogram (SI symbol: kg), also known as the kilo, is the base unit of mass in the International time (t) second (s) second System of Units. (kilogram = 1000 gram(g) ) velocity(v) cm/s m/s acceleration (a) cm/s² m/s² 1 dyne = 1 g·cm/s² = 10−5 kg·m/s² = 10−5 Newton force (F) dyne (dyn) newton (N) 1Newton = 1 kg·m/s2 pressure (p) barye (Ba) pascal (Pa) 1 Ba = 0.1 Pascal = 0.1 Newton/m2 = 1 g·cm-1s-2 2 2 −7 energy (W) erg (erg) joule (J) 1 erg = g·cm /s = 10 joules (1 erg = 1 dyne cm = 1 g·cm2/s2) 1 joule = 107 erg power (P) erg/s watt (W) 1 watt = one joule per second (power measures viscosity (µ) poise (p) Pa·s the rate of energy conversion) 1 poise = 1 g·cm-1·s-1 (1 Pascal·s = 1Pa·s = 1 -1 -1 kg·m ·s (the extent to which a fluid resists a tendency to flow) The International System of Units (System international units or SI) is the current international standard metric system and the system most widely used around the world. Mass can be defined as a quantitative measure of an object's resistance to the change of its speed. -

The Ampère House and the Museum of Electricity, Poleymieux Au Mont D’Or, France (Near Lyon)
The Ampère House The Ampère House and the Museum of Electricity, Poleymieux au Mont d’Or, France (Near Lyon). André-Marie Ampère (1775-1836) Ampere at 21 Ampere at 39 Ampere at 55 Location: Poleymieux au Mont d’Or Compound of the Ampere Family Location: Poleymieux au Mont d’Or Compound of the Ampere Family Educated based on Rousseau theories directly by his father, Jean-Jacques. Never went to school. A genius as soon as 13 years old. A “Prodigy child” learn Latin and other languages. Teach himself the works of Bernouilli and Euler in Latin. Professor of Mathematics, Italian, Chemistry, Mathematics and Physics at 22. Member of the Academy in 1814 (39 years old). Entrance room: History of the Museum Poleymieux au Mont d’Or André-Marie lived there from 7 to 20 years old. His wife and his child stay there a few more years. Museum inaugurated on July 1, 1931. Picture of Hernand & Sosthenes Behn, re-purchased the house to make a museum (Founders of ITT in the USA in 1920). They were from a French Mother and Danish Father. Studied in France and emigrated to New York after graduation. Gave as a gift to the SFE (Société Française des Electriciens) in 1928. Hernand died in France in 1933 in a retirement villa. Room of the Three Amperes. The House of Ampère -Partners Curator: Mr. Georges Asch Plate on the life of Ampere. Definitions (ANSI/IEEE Std 100) Ampere (1) (metric practice). That constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross section, F F and placed at one meter apart in vacuum, would produce between these conductors a force equal to 2x10-7 newton per meter of length (Adopted by the 9th General Conference on Weight and 1 meter Measures in 1948). -

Units of Measurement Course Handouts
AETD MINI-COURSE 115: UNITS OF MEASUREMENT COURSE HANDOUTS D.G. SIMPSON FEBRUARY 2013 GODDARD SPACE FLIGHT CENTER GREENBELT, MARYLAND Contents 1 Metric Prefixes 2 1.1 SI Prefixes............................................ 2 1.2 Computer Prefixes....................................... 3 2SIUnits 4 2.1SIBaseUnits.......................................... 4 2.2SIDerivedUnits(StandardMKSABaseUnits)........................ 5 2.3SIDerivedUnits(MVSABaseUnits)............................. 6 2.4SIEquationsofElectromagnetism............................... 7 3 Electrostatic Units 8 3.1ElectrostaticBaseUnits..................................... 8 3.2ElectrostaticDerivedUnits................................... 9 3.3ElectrostaticEquationsofElectromagnetism.......................... 10 4 Electromagnetic Units 11 4.1ElectromagneticBaseUnits................................... 11 4.2ElectromagneticDerivedUnits................................. 12 4.3ElectromagneticEquationsofElectromagnetism........................ 13 5 Gaussian Units 14 5.1GaussianBaseUnits...................................... 14 5.2GaussianDerivedUnits..................................... 15 5.3GaussianEquationsofElectromagnetism........................... 16 6 Heaviside-Lorentz Units 17 6.1Heaviside-LorentzBaseUnits................................. 17 6.2Heaviside-LorentzDerivedUnits................................ 18 6.3Heaviside-LorentzEquationsofElectromagnetism...................... 19 7 Units of Physical Quantities 20 8 Formula Conversion Table 23 9 Unit Conversion Tables 25 10 Physical