James Clerk Maxwell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Download Report 2010-12
RESEARCH REPORt 2010—2012 MAX-PLANCK-INSTITUT FÜR WISSENSCHAFTSGESCHICHTE Max Planck Institute for the History of Science Cover: Aurora borealis paintings by William Crowder, National Geographic (1947). The International Geophysical Year (1957–8) transformed research on the aurora, one of nature’s most elusive and intensely beautiful phenomena. Aurorae became the center of interest for the big science of powerful rockets, complex satellites and large group efforts to understand the magnetic and charged particle environment of the earth. The auroral visoplot displayed here provided guidance for recording observations in a standardized form, translating the sublime aesthetics of pictorial depictions of aurorae into the mechanical aesthetics of numbers and symbols. Most of the portait photographs were taken by Skúli Sigurdsson RESEARCH REPORT 2010—2012 MAX-PLANCK-INSTITUT FÜR WISSENSCHAFTSGESCHICHTE Max Planck Institute for the History of Science Introduction The Max Planck Institute for the History of Science (MPIWG) is made up of three Departments, each administered by a Director, and several Independent Research Groups, each led for five years by an outstanding junior scholar. Since its foundation in 1994 the MPIWG has investigated fundamental questions of the history of knowl- edge from the Neolithic to the present. The focus has been on the history of the natu- ral sciences, but recent projects have also integrated the history of technology and the history of the human sciences into a more panoramic view of the history of knowl- edge. Of central interest is the emergence of basic categories of scientific thinking and practice as well as their transformation over time: examples include experiment, ob- servation, normalcy, space, evidence, biodiversity or force. -

S.I. and Cgs Units
Some Notes on SI vs. cgs Units by Jason Harlow Last updated Feb. 8, 2011 by Jason Harlow. Introduction Within “The Metric System”, there are actually two separate self-consistent systems. One is the Systme International or SI system, which uses Metres, Kilograms and Seconds for length, mass and time. For this reason it is sometimes called the MKS system. The other system uses centimetres, grams and seconds for length, mass and time. It is most often called the cgs system, and sometimes it is called the Gaussian system or the electrostatic system. Each system has its own set of derived units for force, energy, electric current, etc. Surprisingly, there are important differences in the basic equations of electrodynamics depending on which system you are using! Important textbooks such as Classical Electrodynamics 3e by J.D. Jackson ©1998 by Wiley and Classical Electrodynamics 2e by Hans C. Ohanian ©2006 by Jones & Bartlett use the cgs system in all their presentation and derivations of basic electric and magnetic equations. I think many theorists prefer this system because the equations look “cleaner”. Introduction to Electrodynamics 3e by David J. Griffiths ©1999 by Benjamin Cummings uses the SI system. Here are some examples of units you may encounter, the relevant facts about them, and how they relate to the SI and cgs systems: Force The SI unit for force comes from Newton’s 2nd law, F = ma, and is the Newton or N. 1 N = 1 kg·m/s2. The cgs unit for force comes from the same equation and is called the dyne, or dyn. -
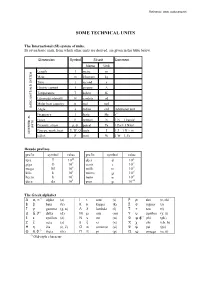
Technical Units
Reference: www. rockmass.net 620(7(&+1,&$/81,76 7KH,QWHUQDWLRQDO 6, V\VWHPRIXQLWV Its seven basic units, from which other units are derived, are given in the table below. Dimension Symbol SI unit Comment Name Unit Length l metre m Mass m kilogram kg Time t second s Electric current I ampere A Temperature T kelvin K Luminous intensity Iv candela cd Molar heat capacity n mol mol BASIC UNITS IN SI SYSTEM Angle a radian rad Additional unit Frequency f hertz Hz s-1 Force F newton N 1 N = 1 kgm/s2 Pressure, stress p, σ, pascal Pa 1 Pa = 1 N/m² UNITS Energy, work, heat E, W, Q joule J 1 J = 1 N × m DIVERTED SI- Effect P watt W 1 W = 1 J/s 'HFDGHSUHIL[HV prefix symbol value prefix symbol value tera T 1012 deci d 10-1 giga G 109 centi c 10-2 mega M 106 milli m 10-3 kilo k 103 micro µ 10-6 hecto h 102 nano n 10-9 deca da 101 pico p 10-12 7KH*UHHNDOSKDEHW Α α, ∝ ∗) alpha (a) Ι ι iota (i) Ρ ρ rho (r, rh) Β β beta (b) Κ κ kappa (k) Σ σ sigma (s) Γ γ gamma (g, n) Λ λ lambda (l) Τ τ tau (t) ∆ δ, ∂ ∗) delta (d) Μ µ mu (m) Υ υ upsilon (y, u) Ε ε epsilon (e) Ν ν nu (n) Φ φ, ϕ*) phi (ph) Ζ ζ zeta (z) Ξ ξ xi (x) X χ chi (ch, h) Η η ëta (e, ë) Ο ο omicron (o) Ψ ψ psi (ps) Θ θ, ϑ ∗) theta (th) Π π pi (p) Ω ω omega (o, õ) *) Old-style character 2 9DULRXVXQLWVDQGFRQYHUVLRQV 'LPHQVLRQ 1DPH 6\PERO 8QLW &RQYHUVLRQ Time minute min s 1 h = 60 s hour h h 1 h = 60 min day day day 1 day = 24 h week wk wk 1 wk = 7 days year yr yr 1 yr = 365.242 days = 52.17 wk Length Angstrom A m 1 A = 10-10 m nautical mile n mile 1 n mile = 1852 m wave length λ m Force load G (P,W) N 1 kp = 9.807 N 2 o o 2 gravity g N 1 gn = 9.80665 m/s (exact, at 45 N; at 60 N g = 9.82 m/s ) atmosphere atm 1 atm Modulus elasticity modulus E Pa 1 kPa = 0.1 N/cm = 0.102 ton/m2 deformation modulus G Pa 1 MPa = 10,2 kg/cm2 shear modulus K Pa Density mass density ρ kg/m3 force density γ kN/m3 Flow Lugeon Lugeon l/min/m 1 Lugeon = approx. -

DIMENSIONS and UNITS to Get the Value of a Quantity in Gaussian Units, Multiply the Value Ex- Pressed in SI Units by the Conversion Factor
DIMENSIONS AND UNITS To get the value of a quantity in Gaussian units, multiply the value ex- pressed in SI units by the conversion factor. Multiples of 3 intheconversion factors result from approximating the speed of light c =2.9979 1010 cm/sec × 3 1010 cm/sec. ≈ × Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units t2q2 Capacitance C l farad 9 1011 cm ml2 × m1/2l3/2 Charge q q coulomb 3 109 statcoulomb t × q m1/2 Charge ρ coulomb 3 103 statcoulomb 3 3/2 density l l t /m3 × /cm3 tq2 l Conductance siemens 9 1011 cm/sec ml2 t × 2 tq 1 9 1 Conductivity σ siemens 9 10 sec− 3 ml t /m × q m1/2l3/2 Current I,i ampere 3 109 statampere t t2 × q m1/2 Current J, j ampere 3 105 statampere 2 1/2 2 density l t l t /m2 × /cm2 m m 3 3 3 Density ρ kg/m 10− g/cm l3 l3 q m1/2 Displacement D coulomb 12π 105 statcoulomb l2 l1/2t /m2 × /cm2 1/2 ml m 1 4 Electric field E volt/m 10− statvolt/cm t2q l1/2t 3 × 2 1/2 1/2 ml m l 1 2 Electro- , volt 10− statvolt 2 motance EmfE t q t 3 × ml2 ml2 Energy U, W joule 107 erg t2 t2 m m Energy w, ϵ joule/m3 10 erg/cm3 2 2 density lt lt 10 Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units ml ml Force F newton 105 dyne t2 t2 1 1 Frequency f, ν hertz 1 hertz t t 2 ml t 1 11 Impedance Z ohm 10− sec/cm tq2 l 9 × 2 2 ml t 1 11 2 Inductance L henry 10− sec /cm q2 l 9 × Length l l l meter (m) 102 centimeter (cm) 1/2 q m 3 Magnetic H ampere– 4π 10− oersted 1/2 intensity lt l t turn/m × ml2 m1/2l3/2 Magnetic flux Φ weber 108 maxwell tq t m m1/2 Magnetic -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

The Astronomical Work of Carl Friedrich Gauss
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector HISTORIA MATHEMATICA 5 (1978), 167-181 THE ASTRONOMICALWORK OF CARL FRIEDRICH GAUSS(17774855) BY ERIC G, FORBES, UNIVERSITY OF EDINBURGH, EDINBURGH EH8 9JY This paper was presented on 3 June 1977 at the Royal Society of Canada's Gauss Symposium at the Ontario Science Centre in Toronto [lj. SUMMARIES Gauss's interest in astronomy dates from his student-days in Gattingen, and was stimulated by his reading of Franz Xavier von Zach's Monatliche Correspondenz... where he first read about Giuseppe Piazzi's discovery of the minor planet Ceres on 1 January 1801. He quickly produced a theory of orbital motion which enabled that faint star-like object to be rediscovered by von Zach and others after it emerged from the rays of the Sun. Von Zach continued to supply him with the observations of contemporary European astronomers from which he was able to improve his theory to such an extent that he could detect the effects of planetary perturbations in distorting the orbit from an elliptical form. To cope with the complexities which these introduced into the calculations of Ceres and more especially the other minor planet Pallas, discovered by Wilhelm Olbers in 1802, Gauss developed a new and more rigorous numerical approach by making use of his mathematical theory of interpolation and his method of least-squares analysis, which was embodied in his famous Theoria motus of 1809. His laborious researches on the theory of Pallas's motion, in whi::h he enlisted the help of several former students, provided the framework of a new mathematical formu- lation of the problem whose solution can now be easily effected thanks to modern computational techniques. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

OH Absorption Spectroscopy to Investigate Light
OH Absorption Spectroscopy to Investigate Light- Load HCCI Combustion by Sean J. Younger A thesis submitted in partial fulfillment of the requirements for a degree of Master of Science (Mechanical Engineering) at the University of Wisconsin – Madison 2005 Approved: ____________________________ Jaal B. Ghandhi Professor – Mechanical Engineering University of Wisconsin – Madison Date: ________________________ i Abstract Absorption spectroscopy of the OH molecule was used to examine the light-load limit of the HCCI combustion process. Optical results were compared to cylinder pressure and emissions data, with a focus on the transition from low to high engine-out CO emissions. The goal of the work was to experimentally verify a low-load emissions limit to practical HCCI combustion that has been predicted by detailed kinetic simulations. Two diluent mixtures were used along with 100% air to create intake charges with varying specific heats in order to highlight the temperature dependence of OH formation. Peak OH concentration data show a correlation with temperature, which agrees with theory. In general, OH absorption decreased monotonically with the mass of fuel injected per cycle for all diluent cases. The absorption spectra, which were taken with 400 µs time resolution (~1.8 CAD at 600 RPM) show that OH forms during the second- stage heat release and remains in the cylinder well into the expansion stroke. Spectral resolution did not allow a temperature measurement from absorption, so temperature was measured separately. Emissions data showed low exhaust CO concentrations at high fuel rates, and higher CO concentrations at low fueling rates. Qualitatively, the detection limit for OH coincided with the onset of increased engine-out CO emissions and the transition from strong to weak second stage heat release. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

John Wallis (1616–1703), Oxford's Savilian Professor
John Wallis (1616–1703), Oxford’s Savilian Professor of Geometry from 1649 to 1703, was the most influential English mathematician before the rise of Isaac Newton. His most important works were his Arithmetic of Infinitesimals and his treatise on Conic Sections, both published in the 1650s. It was through studying the former that Newton came to discover his version of the binomial theorem. Wallis’s last great mathematical work A Treatise of Algebra was published in his seventieth year. John Wallis Wallis’ time-line “In the year 1649 I removed to 1616 Born in Ashford, Kent Oxford, being then Publick 1632–40 Studied at Emmanuel College, Professor of Geometry, of the Cambridge Foundation of Sr. Henry Savile. 1640 Ordained a priest in the And Mathematicks which Church of England had before been a pleasing 1642 Started deciphering secret codes diversion, was now to be for Oliver Cromwell’s intelligence my serious Study.” service during the English Civil Wars John Wallis 1647 Inspired by William Oughtred’s Clavis Mathematicae (Key to Mathematics) which considerably extended his mathematical knowledge 1648 Initiated mathematical correspondence with continental scholars (Hevelius, van Schooten, etc.) 1649 Appointed Savilian Professor of Geometry at Oxford 31 October: Inaugural lecture 1655–56 Arithmetica Infinitorum (The Arithmetic of Infinitesimals) and De Sectionibus Conicis (On Conic Sections) 1658 Elected Oxford University Archivist 1663 11 July: Lecture on Euclid’s parallel postulate 1655–79 Disputes with Thomas Hobbes 1685 Treatise of Algebra 1693–99 Opera Mathematica 1701 Appointed England’s first official decipherer (alongside his grandson William Blencowe) 1703 Died in Oxford John Wallis (1616–1703) Savilian Professor During the English Civil Wars John Wallis was appointed Savilian Professor of Geometry in 1649. -

Hyperbolic Geometry in the Work of Johann Heinrich Lambert Athanase Papadopoulos, Guillaume Théret
Hyperbolic geometry in the work of Johann Heinrich Lambert Athanase Papadopoulos, Guillaume Théret To cite this version: Athanase Papadopoulos, Guillaume Théret. Hyperbolic geometry in the work of Johann Heinrich Lambert. Ganita Bharati (Indian Mathematics): Journal of the Indian Society for History of Mathe- matics, 2014, 36 (2), p. 129-155. hal-01123965 HAL Id: hal-01123965 https://hal.archives-ouvertes.fr/hal-01123965 Submitted on 5 Mar 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. HYPERBOLIC GEOMETRY IN THE WORK OF J. H. LAMBERT ATHANASE PAPADOPOULOS AND GUILLAUME THERET´ Abstract. The memoir Theorie der Parallellinien (1766) by Jo- hann Heinrich Lambert is one of the founding texts of hyperbolic geometry, even though its author’s aim was, like many of his pre- decessors’, to prove that such a geometry does not exist. In fact, Lambert developed his theory with the hope of finding a contra- diction in a geometry where all the Euclidean axioms are kept except the parallel axiom and that the latter is replaced by its negation. In doing so, he obtained several fundamental results of hyperbolic geometry. This was sixty years before the first writings of Lobachevsky and Bolyai appeared in print.