Guidance for TB Notification in India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nss Activities 2017-18 Sl
NSS ACTIVITIES 2017-18 SL. DATE PLACE PROGRAMME ORGANISED BY NO ORGANISED AND SPEAKER 1 01.05.2017 Yendurance, “National Integration Camp National Youth Project&NSS to YenepoyaUniversity for Youth” Unit of Yenepoya university 07.05.2017 campus 2 03.08.2017 The Yenepoya The Inaugural Function The Yenepoya Institute of Arts, Institute of Arts, Science NSS Wing of the Science College 3 12.08.2017 Olavinahalli, kinya Old age home named The Yenepoya Institute of Arts, Deralakatte “Olavinahalli” Science NSS Wing of the College 4 12.09.2017 Yenepoya Nursing Environment Programme NSS Unit -Yenepoya Nursing college Speaker -Dr. Madhu Manohar College –Environmental Engineer 5 24.09.2017 Nehru Maidan Keep Mangalore clean Yenepoya Nursing College Programme 6 29.09.2017 Mumbai Thakur “Cancer Awarness National Service Scheme – NSS Polytechnic Outreach Programme Unit- MH 09B45 – Mumbai 7 01.10.2017 Barpole Coorg “Adventure Camp”River Ministry of Youths Affairs & District rafting training for Sports Govt of India volunteers 8 05.10.2017 Yenepoya “ Swacchta hi seva” NSS Unit of Yenepoya Medical University College 9 10.10.2017 Pajjiru Nadu village “Swachhta Pakwara Yenepoya Dental College NSS Programme” Unit 10 13.10.2017 The Yenepoya “Blood Grouping &General The NSS Wing of the College Institute of Arts, Health Checkup” Rural Health &Community Science college Development Auditorium 11 21.10.2017 Karnal Hariyana “Internatinal Youth NIFAA, The National Integrated to Festival” Forum for Artists & Activists 29.10.2017 12 04.11.2017 Tippu Sulthan P.U “Blood -

Project Management Consultancy for Implementation of Smart City Mission Projects for Mangaluru City
APPOINTMENT OF PROJECT MANAGEMENT CONSULTANTS FOR IMPLEMENTATION OF SMART CITY MISSION PROJECTS IN MANGALURU CITY 2018 DETAILED PROJECT REPORT – CONVERSION OF ALL LIGHTING IN GOVERNMENT BUILDINGS (IN ABD AREA) INTO LED PROJECT MANAGEMENT CONSULTANCY FOR IMPLEMENTATION OF SMART CITY MISSION PROJECTS FOR MANGALURU CITY DETAILED PROJECT REPORT ON CONVERSION OF ALL LIGHTING IN GOVERNMENT BUILDINGS IN ABD INTO LED LIGHTING The purpose of the Detailed Project Report is to provide details of various considerations made towards the elements proposed for the project as mentioned in the title above. It aims to give a basic design idea to all the stakeholders before proceeding for final design and estimates. | P a g e Mangaluru Smart City Limited (MSCL) APPOINTMENT OF PROJECT MANAGEMENT CONSULTANTS FOR IMPLEMENTATION OF SMART CITY MISSION PROJECTS IN MANGALURU CITY DETAILED PROJECT REPORT – CONVERSION OF ALL LIGHTING IN GOVERNMENT BUILDINGS (IN ABD AREA) INTO LED ISSUE AND REVISION RECORD Revision Date Originator Checker Approver Description Standard 0 26/03/2018 WTESL/LBI/C URVI BHATT URVI BHATT/ Detailed DAC KAVITA Project WAKADE Report 1 24/08/2018 WTESL/LBI/CD Dr. Nitin URVI BHATT/ Detailed Project AC Bhavsar KAVITA Report WAKADE Mangaluru Smart City Limited (MSCL) i | P a g e APPOINTMENT OF PROJECT MANAGEMENT CONSULTANTS FOR IMPLEMENTATION OF SMART CITY MISSION PROJECTS IN MANGALURU CITY DETAILED PROJECT REPORT – CONVERSION OF ALL LIGHTING IN GOVERNMENT BUILDINGS (IN ABD AREA) INTO LED This document is issued for the party which We accept no responsibility for the commissioned it and for specific purposes consequences of this document being relied connected with the above-captioned project upon by any other party, or being used for any only. -

World Bank Document
EXECUTIVE SUMMARY 1.0 Mizoram State Road Project II (MSTP II) The Government of India has requested World Bank for financing rehabilitation, widening Public Disclosure Authorized and strengthening of State Highways and District Roads in the State of Mizoram, and enhances connectivity. In line with this request, Mizoram State Roads Project II (MSRP II) is proposed. The proposed roads under MSRP II are shown in Map-1. The MSRP II is to be implemented in two groups. The proposed group –I and group –II project corridors are shown in figure 1 and table 1. Group –I of the project is under project preparation. Project Preparatory Consultants1 (PPC) is assisting MPWD in project preparation. The MSR II has been categorised as category ‘A’ project. Table 1.1 – Proposed Project Roads under MSRP II Group -1 District(s) Length i. Champhai – Zokhawthar Champhai 27.5 km, (E-W road to Myanmar Public Disclosure Authorized border) ii Chhumkhum-Chawngte Lunglei 41.53 km, (part of original N-S road alignment) Group – 2 i. Lunglei - Tlabung - Lunglei 87.9 km, (E-W road to Bangladesh Kawrpuichhuah border) ii. Junction NH44A (Origination) – Mamit&Lunglei 83 km Chungtlang – Darlung – Buarpui iii. Buarpui – Thenlum – Zawlpui Lunglei 95 km iv Chawngte including bridge to Lawngtlai 76 km Public Disclosure Authorized BungtlangSouth up to Multimodal Road junction v. Zawlpui – Phairuangkai Lunglei 30 km 1.1 Champhai – Zokhawthar road The Mizoram Public Works Department has decided to upgrade the existing 28.5 km Champhai – Zokhawthar road from single road state highway standard to 2-Lane National Highway Standard. This road passes through a number of villages like Zotlang, Mualkwai, Melbuk Zokhawthar and part of Champhai town etc. -

A Baseline Survey of Minority Concentration Districts of India
A BASELINE SURVEY OF MINORITY CONCENTRATION DISTRICTS OF INDIA Lawngtlai (Mizoram) Sponsored by Ministry of Miniority Affairs Government of India and Indian Council of Social Science Research INSTITUTE FOR HUMAN DEVELOPMENT NIDM Building, 3rd Floor, IIPA Campus I.P Estate, Mahatma Gandhi Marg, New Delhi-110 002 Phones – 2335 8166, 2332 1610 / Fax : 23765410 Email: [email protected], website:ihdindia.org 2008 A BASELINE SURVEY OF MINORITY CONCENTRATION DISTRICTS OF INDIA Lawngtlai (Mizoram) Sponsored by Ministry of Miniority Affairs Government of India and Indian Council of Social Science Research INSTITUTE FOR HUMAN DEVELOPMENT NIDM Building, 3rd Floor, IIPA Campus I.P Estate, Mahatma Gandhi Marg, New Delhi-110 002 Phones – 2335 8166, 2332 1610 / Fax: 23765410 Email: [email protected], website: www.ihdindia.org RESEARCH TEAM Principal Researchers Alakh N. Sharma Ashok K. Pankaj Data Processing and Tabulation Balwant Singh Mehta Sunil Kumar Mishra Abhay Kumar Research Associates/Field Supervisors Ramashray Singh Ashwani Kumar Subodh Kumar M. Poornima Research Assistant P.K. Mishra Secretarial Assistance Shri Prakash Sharma Nidhi Sharma Sindhu Joshi LAWNGTLAI Principal Authors of the Report Falendra K. Sudan Senior Visiting Fellow, IHD & R.P.Mamgain Senior Fellow, IHD CONTENTS Executive Summary....................................................................................................i-vii Chapter I: Introduction ..............................................................................................1-10 Chapter II: Village Level Deficits.............................................................................11-15 -
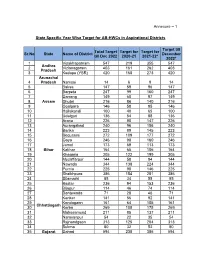
Annexure – 1 State Specific Year Wise Target for AB-Hwcs In
Annexure – 1 State Specific Year Wise Target for AB-HWCs in Aspirational Districts Target till Total Target Target for Target for Sr.No State Name of District December till Dec 2022 2020-21 2021-22* 2022* 1 Visakhapatnam 547 219 355 547 Andhra 2 Vizianagaram 403 161 262 403 Pradesh 3 Kadapa (YSR) 420 168 273 420 Arunachal 4 Pradesh Namsai 14 6 9 14 5 Baksa 147 59 96 147 6 Barpeta 247 99 160 247 7 Darrang 149 60 97 149 8 Assam Dhubri 216 86 140 216 9 Goalpara 146 58 95 146 10 Hailakandi 100 40 65 100 11 Udalguri 136 54 88 136 12 Araria 226 90 147 226 13 Aurangabad 240 96 156 240 14 Banka 223 89 145 223 15 Begusarai 272 109 177 272 16 Gaya 246 98 160 246 17 Jamui 173 69 113 173 18 Bihar Katihar 164 65 106 164 19 Khagaria 305 122 199 305 20 Muzaffarpur 144 58 94 144 21 Nawada 344 138 224 344 22 Purnia 225 90 146 225 23 Sheikhpura 386 154 251 386 24 Sitamarhi 85 34 55 85 25 Bastar 236 94 153 236 26 Bijapur 114 46 74 114 27 Dantewada 71 28 46 71 28 Kanker 141 56 92 141 29 Kondagaon 161 64 105 161 Chhattisgarh 30 Korba 269 108 175 269 31 Mahasamund 211 85 137 211 32 Narayanpur 54 22 35 54 33 Rajnandgaon 313 125 204 313 34 Sukma 80 32 52 80 35 Gujarat Dahod 594 238 386 594 Target till Total Target Target for Target for Sr.No State Name of District December till Dec 2022 2020-21 2021-22* 2022* 36 Narmada 162 65 106 162 37 Haryana Nuh (Mewat) 88 35 57 88 Himachal 38 Pradesh Chamba 167 67 108 167 39 Jammu & Baramulla 204 82 133 204 40 Kashmir Kupwara 241 97 157 241 41 Bokaro 107 43 70 107 42 Chatra 86 35 56 86 43 Dumka 232 93 150 232 44 Garhwa -

Resutls for Gramin Dak Sevak for North Eastern Circle
Resutls for Gramin Dak Sevak for North Eastern Circle S.No Division HO Name SO Name BO Name Post Name Cate No Registration Selected Candidate gory of Number with Percentage Post s 1 Manipur Imphal H.O Chakpikarong Gobok B.O GDS BPM UR 1 R1DC82FB64C36 SOWNDERYA M S.O (92.8) 2 Manipur Imphal H.O Chakpikarong Sajik Tampak GDS MD UR 1 R018457C4E49D THIYAM INAO S.O B.O SINGH (91.2) 3 Manipur Imphal H.O Chandel S.O Mittong B.O GDS BPM UR 1 R4C18B72F4591 KHUTLEIBAM MD KANOON HAQUE (91.8) 4 Manipur Imphal H.O Churachandp South Kotlein GDS BPM UR 1 R84F8FBA8DA21 PUKHRAMBAM ur S.O B.O VASKAR SINGH (91.2) 5 Manipur Imphal H.O Lamlong S.O Huidrom B.O GDS MD OBC 1 R24756BB59EAD SANGEETH M P (92.8889) 6 Manipur Imphal H.O Lamlong S.O Nongoda B.O GDS BPM SC 1 R1AC74CAF5489 CHINGTHAM LOYALAKPA SINGH (95) 7 Manipur Imphal H.O Litan S.O Semol B.O GDS BPM UR 1 R8723E884ED43 RUHANI ALI (91.2) 8 Manipur Imphal H.O Litan S.O Thawai B.O GDS BPM UR 1 R3A6764E447F4 SHYJIMOL C (94.6) 9 Manipur Imphal H.O Mao S.O Mao S.O GDS MD UR 1 R74DBE3D94C4B MD SARIF (91.2) 10 Manipur Imphal H.O Moreh S.O Bongjoi B.O GDS BPM UR 1 R5DB3F6B4AAF3 MD YAKIR HUSSAIN (91.2) 11 Manipur Imphal H.O Nambol S.O Bungte Chiru GDS BPM UR 1 R52EAF9243942 THOUDAM B.O MICHEAL SINGH (95) 12 Manipur Imphal H.O Nambol S.O Manamayang GDS BPM UR 1 R7AFF761A47A4 KUMAR MANGLAM B.O (93.1) 13 Manipur Imphal H.O Singhat S.O Behiang B.O GDS BPM UR 1 R53B454F6511B RITEZH THOUDAM (95) 14 Manipur Imphal H.O Yairipok S.O Angtha B.O GDS BPM OBC 1 R03184F4ECDC8 KOHILA DEVI G (95) 15 Manipur Imphal H.O Yairipok -

5. Secretary, Minority Welfare Department, Uttarkhand.
No. 31298/2017-MsDP Government of India Ministry of Minority Affairs 11th Floor, Pt. Deen Dayal Antodaya Ilhavan, C.G.O. Complex, Lodhi Road, New Delhi — 110003 Dated: 24 .11.2017 OFFICE MEMORANDUM Subject: MINUTES OF THE 135' MEETING OF EMPOWERED COMMITTEE UNDER MULTI-SECTORAL DEVELOPMENT PROGRAMME HELD ON 23.11.2017 UNDER TIM CHAIRMANSHIP OF SECIUTARY, MINISTRY OF MINORITY AFFAIRS.. 'Hie undersigned is directed to forward herewith Minutes of the 135 th meeting of Empowered Committee under Multi-Sectoral Development Programme held on 2111.2017 under the Chairmanship of Secretary, Ministry of Minority Affairs for necessary action. (Geeta Under Secretary to the Govt. of India To: I. Ministry of Finance, {Secretary, Department of Expenditure), North Mock. New Delhi 2. Advisor (Social Sector), Planning Commission, Yojana Bhawan. New Delhi 3. Secretary, Ministry of Women & Child Development, Shastri Rhawan, New Delhi. 4. Secretary, Ministry of Labour & Employment, Shram Shakti Rhawan, New Delhi. 5, Secretary, Ministry of Rural Development, Krishi Bhawan, New Delhi. 6, Secretary. Department of Drinking Water Supply, CG() Complex, New Delhi. 7, Secretary, Ministry of Health & Faniil Welfare, Nirman Bhawan, New Delhi, 8. Secretary, Department of higher Education, Shastri Rhawan, New Delhi. 9, Secretary, Department of School Education and literacy, Shastri Bhawan. New Delhi 10, Secretary, Ministry of Skill Development, Shivaji Stadium, New Delhi. Copy for information:- 1. Secretary, Minority & other backward classes, Manipur 2. Pr. Secretary, Minority Welfare Department, Bihar 3. Secretary, Social Justice & Minorities Department, Haryana 4. Secretary, Minorities Welfare Department, Govt. of Mizoram 5. Secretary, Minority Welfare Department, Uttarkhand. F .13/ 298/ 2frl 7- Ms DP-MoMA Govt. -

Government of India Ministry of Health and Family Welfare Department of Health and Family Welfare
GOVERNMENT OF INDIA MINISTRY OF HEALTH AND FAMILY WELFARE DEPARTMENT OF HEALTH AND FAMILY WELFARE LOK SABHA STARRED QUESTION NO.178 TO BE ANSWERED ON THE 21ST DEEMBER, 2018 RANKING OF DISTRICT HOSPITALS *178. DR. KIRIT SOMAIYA: Will the Minister of HEALTH AND FAMILY WELFARE be pleased to state: (a) the total number of district hospitals in the country, State and districtwise; (b) whether the Ministry along with NITI Aayog has started ranking the district hospitals and if so, the details thereof; (c) the details of criteria, parameters, indicators, etc. followed in ranking district hospitals; (d) the timeframe to complete the ranking process of these hospitals; and (e) the manner in which the general public and patients will be benefited by this ranking? ANSWER THE MINISTER OF HEALTH AND FAMILY WELFARE (SHRI JAGAT PRAKASH NADDA) (a) to (e) : A Statement is laid on the Table of the House. STATEMENT REFERRED TO IN REPLY TO LOK SABHA STARRED QUESTION NO.178* FOR 21ST DECEMBER, 2018 (a): Total number of district hospitals in the country, State and district wise is given at Annexure. (b): Ministry of Health and Family Welfare along with the NITI Aayog, has not started the ranking of District Hospitals. (c): A conceptual framework has been developed by NITI Aayog in consultation with the Ministry of Health and Family Welfare to track performance of District Hospitals. The link of the framework indicating the criteria, parameters, indicators etc. is available on the link at http://niti.gov.in /content/tracking-performance- district-hospitals-health-our-hospitals. (d): The process for ranking of the District Hospitals is to be done on an annual basis. -

Friday 26 July 2019
Friday 26th July 2019 (For the period 26th July to 30th July 2019) Weblink For District AAS Bulletin: http://www.imdagrimet.gov.in/node/3545 State Composite AAS Bulletin: http://www.imdagrimet.gov.in/node/3544 1 Spatial distribution of weather parameters for the week ending on 24.07.2019 Mean Maximum Temperature (oC) for the week Mean Minimum Temperature (oC) for the week ending on 24.07.2019 ending on 24.07.2019 Mean Diurnal Temperature Variation (oC) for the Mean Maximum Relative Humidity (%) for the week week ending on 24.07.2019 ending on 24.07.2019 Mean Minimum Relative Humidity (%) for the week Mean Wind Speed (km/hr) for the week ending on ending on 24.07.2019 24.07.2019 Mean Cloud Cover (okta) for the week ending on 24.07.2019 2 NOAA/VIIRS/BLENDED NDVI composite for the week Difference in NOAA/VIIRS/BLENDED NDVI number 29 (15.07.2019 to 21.07.2019) over Agricultural between week number 29 and week number 28 regions of India Agriculture vigour was good at many places over Good agricultural progress was observed over valley region of Jammu & Kashmir and in isolated places in Madhya Pradesh, Konkan, East Nagappattinam district of Tamilnadu. Moderate Vidarbha, Chattisgarh, Jharkhand, North vigour was observed at isolated places of North Telangan, North Coastal Andhra Pradesh and Telangana, North Coastal Andhra Pradesh, Odisha, Uttarakhand. It was moderate over rest of the North Eastern states, Jharkhand, West Bengal, country. Western part of Uttar Pardesh, Himachal Pradesh, Uttarakhand, South Interior Karnataka and East Rajashthan. NDVI could not be derived over rest of the country due to persistant cloud cover. -

In the High Court of Karnataka at Bangalore
1 IN THE HIGH COURT OF KARNATAKA AT BANGALORE DATED THIS THE 23 RD DAY OF JULY 2013 PRESENT THE HON'BLE MR.JUSTICE K.L.MANJUNATH AND THE HON’BLE MR.JUSTICE RAVI MALIMATH WRIT APPEAL No.3949/2010 (S-RES) AND WRIT APPEAL Nos.4332-4349/2010 (S-RES) C/W WRIT APPEAL Nos.4015-4033/2010 (S-RES) IN WRIT APPEAL No.3949/2010(S-RES): & WA Nos.4332-4349/2010(S-RES): BETWEEN: 1. AMBARISH M.R S/O REVAPPA GOWDA AGED ABOUT 27 YEARS C/O B.Y.SHANMUKAPPA, H.NO.EB.60(1-1) MESCOM GUEST HOUSE KUNJIBETTU, UDUPI-576102. 2. K.SURESH SHETTY, S/O T.SRIDHAR SHETTY, AGED ABOUT 39 YEARS KEDOOR HEBBADI HOUSE, KEDOOR POST AND VILLAGE KUNDAPUR TALUK UDUPI DISTRICT-576231. 2 3. HARISH, S/O BOODA POOJARY AGED ABOUT 35 YEARS MOODU VILLAGE ELLINJE POST MANGALORE TALUK D.K.DISTRICT-574141. 4. BASAPPA HADAPADA, S/O YELLAPPA HADAPADA, AGED ABOUT 32 YEARS MESCOM THOTA, UDUPI TALUK AND DISTRICT-576101. 5. DINESH KUMAR S/O ANANDA POOJARY AGED ABOUT 30 YEARS ARPITH NIWAS, JARKAL, KUKKUNDOOR POST, KARKALA TALUK UDUPI DISTRICT-576117 6. RAJESH ACHAR, S/O MANJUNATH ACHAR, AGED ABOUT 31 YEARS MARGOKI BARUR POST, KUNDAPUR TALUK, UDUPI DISTRICT-576231 7. NAGARAJA, S/O RAMAPPA, AGED ABOUT 35 YEARS SAIBRUKATTE YADTHADY POST AND VILLAGE SAIBRA DATTE, UDUPI TALUK AND DISTRICT-576101. 3 8. SANTHOSH V S/O NARASIMHA BILLVA AGED ABOUT 31 YEARS SANTHOSH NILAYA VAKKERY, POST BADAKERE NAVANDA, KUNDAPURA TALUK, UDUPI DISTRICT-576231. 9. UDAYA KUMAR S/O K.SUDHAKARAM, AGED ABOUT 32 YEARS "SUDHA SADANAM" ALBADY VILLAGE, BELVE POST, KUNDAPUR TALUK UDUPI DISTRICT-576212. -

Land Use Planning for Lawngtlai District, Mizoram, India: a Remote Sensing and GIS Perspective
ISSN: 2347-3215 Volume 2 Number 3 (March-2014) pp. 42-53 www.ijcrar.com Land Use Planning for Lawngtlai District, Mizoram, India: A Remote Sensing and GIS perspective R.K.Lallianthanga*, Robert Lalchhanhima Sailo, Hmingthanpuii and H.Lalhmachhuana Mizoram Remote Sensing Application Centre, Science & Technology, Aizawl, Mizoram, India *Corresponding author KEYWORDS A B S T R A C T Land development and its consequent use has a large impact on its immediate GIS; environment and sustainability. Hence, land use planning forms an important Lawngtlai; core component in development programmes of hilly areas for evolving Land Use plan; effective plans at regional level. Hilly terrains offer a lot of challenges in land Remote sensing. use planning due to their intricate bio-physical and socio-economic setup. Hence, the integration of scientific input through advanced techniques are required. The north-eastern part of India are well known for its inherent practice of shifting cultivation which have rendered considerable destruction to both land resources and environment. There is a necessity to develop proper strategies or land use plans which can counteract these detrimental effects on environment, and at the same time improve productivity of land. This study deals with the application of remote sensing and GIS for land use planning in Lawngtlai district of Mizoram, India. Indian Remote Sensing satellite data (LISS-III and Cartosat-I) has been used for generating various GIS layers like land use, slope, soil, drainage, etc. Integration of these with collected ground data resulted in generation of a comprehensive land use plan for the study area. The analysis in a GIS system helped in bringing out maps and statistics with constructive options for alternate land use plans which are both productive and sustainable. -

Sector-Wise Monthly Report on Status of Funding of NEC Projects for the Month of August, 2018
Sector-wise Monthly Report on Status of Funding of NEC Projects for the month of August, 2018 Sl. Sanction Approved NEC's share State's share No. date Cost Delay in State State Utilization NEC Release NEC Release Utilization %age Schedule date compl- share share Major NEC Project Receive State Sector (Date) (Amount) Receive (Date) Utilization of completion etion release release Head (Amount) (Months) (Amount) (%) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Alternative Model of the Shifting Cultivation at Rajen Happa of Ruhi, Tagungpung, Jaapug, Tamuk, Lota 1 and Sartam Village under Tali 21-11-2013 400.00 360.00 40.00 21-11-2013 144.00 22-12-2014 144.00 November, 2016 21 0.00 ArunachalAgri Pradesh & AlliedMH-3601 Circle, Kurung Kumey District, Arunachal Pradesh (Location: Kurung Kumey dist) 400.00 360.00 40.00 144.00 144.00 100.00% 0.00 0.00% ArunachalAgri Pradesh & AlliedMH-3601 20-11-2013 144.00 01-09-2014 144.00 ArunachalAgri Pradesh & AlliedMH-3601 Establishment of Orange cum Pineapple Garden at Kamrung Hotka of Pech Hoj village under 2 20-11-2013 400.00 360.00 40.00 November, 2016 21 0.00 Toru Circle of Papumpare District, 15-12-2014 144.00 07-08-2017 144.00 ArunachalAgri Pradesh & AlliedMH-3601 Arunachal Pradesh (Location: Papum Pare dist) 400.00 360.00 40.00 288.00 288.00 100.00% 0.00 0.00% ArunachalAgri Pradesh & AlliedMH-3601 15-02-2014 90.00 20-01-2015 90.00 ArunachalAgri Pradesh & AlliedMH-3601 Cultivation of large Cardamom at Khakam, Longchan, Wakka and 3 Pongchau under Longding District, 15-02-2014 500.00 450.00 50.00 February,