Barium and Barium Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barium Acetate
Barium acetate sc-202968 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Barium acetate STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH2 HAZARD INSTABILITY0 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Chemical reagent, acetates, mordant for printing fabrics, catalyst manufacturing, paint and varnish dryers, lubricating oil and grease additive. SYNONYMS C4-H6-O4-Ba, Ba(C2H3O2)2, "barium diacetate", "acetic acid barium salt", "octan barnaty" Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability: 1 Toxicity: 2 Body Contact: 2 Min/Nil=0 Low=1 Reactivity: 1 Moderate=2 High=3 Chronic: 2 Extreme=4 CANADIAN WHMIS SYMBOLS 1 of 12 EMERGENCY OVERVIEW RISK Harmful by inhalation and if swallowed. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ! Accidental ingestion of the material may be harmful; animal experiments indicate that ingestion of less than 150 gram may be fatal or may produce serious damage to the health of the individual. ! Ingestion of soluble barium compounds may result in ulceration of the mucous membranes of the gastrointestinal tract, tightness in the muscles of the face and neck, gastroenteritis, vomiting, diarrhea, muscular tremors and paralysis, anxiety, weakness, labored breathing, cardiac irregularity due to contractions of smooth striated and cardiac muscles (often violent and painful), slow irregular pulse, hypertension, convulsions and respiratory failure. -

Barium, Inorganic Water-Soluble Compounds
Barium, inorganic water-soluble compounds Evaluation of health hazards and proposal of health based quality criteria for soil and drinking water Environmental Project No. 1516, 2013 Title: Author: Barium, inorganic water-soluble compounds. Elsa Nielsen Evaluation of health hazards and proposal of Ole Ladefoged health based quality criteria for soil and drinking Division of Toxicology and Risk Assessment water National Food Institute, Technical University of Denmark Published by: The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. Authored in 2006 978-87-93026-71-1 Published in 2013 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning research and development projects within the environmental sector, financed by study grants provided by the Danish Environmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. Sources must be acknowledged. Content CONTENT 2 PREFACE 5 1 GENERAL DESCRIPTION 6 1.1 IDENTITY 6 1.2 PHYSICAL / CHEMICAL PROPERTIES 6 1.3 PRODUCTION AND USE 7 1.4 ENVIRONMENTAL OCCURRENCE 7 1.4.1 Air 7 1.4.2 Water 7 1.4.3 Soil 8 1.4.4 Foodstuffs 8 1.5 ENVIRONMENTAL FATE 8 1.5.1 Air 8 1.5.2 -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

RFC: IRIS Barium and Compounds Substance File
October 29, 2002 Information Quality Guidelines Staff Mail Code 28221T U.S. EPA 1200 Pennsylvania Ave., N.W. Washington, DC, 20460 Subject: Request for Correction of the IRIS Barium and Compounds substance file - Information disseminated by EPA that does not comply with EPA or OMB Information Quality Guidelines Dear Madam or Sir; Chemical Products Corporation (CPC), a Georgia corporation which produces Barium and Strontium chemicals at its Cartersville, Georgia facility, hereby submits this Request for Correction (RFC) concerning EPA’s Integrated Risk Information System Barium and Compounds Substance File (IRIS Ba File). The influential information contained in this file fails to comply with the OMB “Guidelines for Ensuring and Maximizing the Quality, Objectivity, Utility, and Integrity of Information Disseminated by Federal Agencies”. The information disseminated in EPA’s IRIS Barium and Compounds file directly contradicts the information published by EPA in the January 3, 1997 Federal Register and, therefore, cannot represent an EPA consensus position. The IRIS Ba File was revised in 1998 and 1999, yet it contains no mention of the toxicological evaluation conducted by EPA’s Office of Pollution, Pesticides, and Toxic Substances reported in 62 FR 366-372 (No. 2, January 3, 1997). There is no explanation of how a radically different interpretation of the same data could be justified. The NOAEL employed to calculate the Oral Reference Dose in the IRIS Ba File is 0.21 mg/kg/day; there is no LOAEL associated with this NOAEL. The NOAEL reported in 62 FR 366-372 is 70 mg/kg/day in rats and 165 mg/kg/day in mice; these values are taken from a National Toxicology Program technical report and are associated with a LOAEL of 180 mg/kg/day. -

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

The Chemistry of Strontium and Barium Scales
Association of Water Technologies October 20 -23, 2010 Reno, NV, USA The Chemistry of Strontium and Barium Scales Robert J. Ferguson and Baron R. Ferguson French Creek Software, Inc. Kimberton, PA 19442 (610) 935-8337 (610) 935-1008 FAX [email protected] [email protected] Abstract New ‘mystery scales’ are being encountered as cooling tower operators increase cycles to new highs, add ‘reuse’ water to the make-up, and utilize new make-up water sources as part of an overall water conservation strategy. Scales rarely, if ever, encountered in the past are emerging as potential problems. This threat of unexpected scale is compounded because most water treatment service companies do not include barium and strontium in their make-up water analyses. Water sources with even as little 0.01 mg/L of Ba (as Ba) can become very scale-forming with respect to barite (BaSO4) when tower concentration ratios are increased and sulfuric acid used for pH control. Make-up waters incorporating reverse osmosis concentrate can also provide a strontium and barium source. In some cases, produced waters are also being used in an effort for greener water use. This paper discusses the chemistry of the barium and strontium based scales barite (BaSO4), celestite (SrSO4), witherite (BaCO3) and strontianite (SrCO3). Conditions for formation and control from a water treater’s perspective are emphasized. Indices for prediction are discussed. Scale Prediction and the Concept of Saturation A majority of the indices used routinely by water treatment chemists are derived from the basic concept of saturation. A water is said to be saturated with a compound (e.g. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Thermoplastic Composite Chemical Compatibility Guide
123 WR ®300/525/600, AR ®HT & ARLON ® 1000 Chemical Resistance Data WR®300/525, AR®HT Concentrate & ARLON® 1000 Chemical Weight % <275°F (135°C) >275°F (135°C) WR®600 Comments Acetaldehyde, aq. 40 111 Acetamide, aq. 50 111 Acetic Acid, aq. 10 111 Acetic Acid, glacial 100 121 Acetic Anhydride 11Material 1 may react with absorbed moisture Acetone 1 2 – 3 1 Mechanical properties loss <30% for material 2 at T>120 ºF (50 ºC) Acetonitrile 11 Acetophenone 2 3 – 4 1 Material 1 may be slightly soluble at temperatures >390ºF (200ºC) Acetylene 11 Acetyl Chloride, dry 11Material 1 may react with absorbed moisture Acids-Aliphatic, pure 1 1 – 2 1 Acids-Sulfonic, pure 2 – 4 3 – 4 1 Acids-Non oxidizing, aq. <40 1 1 – 2 1 Acrylic Acid 111 Acrylonitrile 11 Adipic Acid 11 Air 1 2 – 3 1 Oxidation for material 1 increases at higher temperatures Alcohols, Aliphatic 111 Allyl Chloride 2 2 – 3 1 Allyl Alcohol 111 Aluminum Chloride, aq. 10 111 Aluminum Chloride, anydrous 441 Aluminum Nitrate, aq. Sat. 11 Aluminum Sulfate, aq. 10 111 Ammonia, aq. Conc. 111 Ammonia, Liquid 11 Ammonia Gas 111 Ammonium Carbonate, aq. 10 111 Ammonium Chloride, aq. 10 111 Ammonium Chloride, aq. 37 111 Ammonium Fluoride, aq. Sat. 11 Ammonium Hydroxide 11 Notes: Material 1= WR300, WR525, ARHT & Arlon 1000 Material 2= WR600 www.gtweed.com WR®300/525, AR®HT Concentrate & ARLON® 1000 Chemical Weight % <275°F (135°C) >275°F (135°C) WR®600 Comments Ammonium Nitrate, aq. Sat. 11 Ammonium Phosphate, aq. Sat. -

Strontium Sulfate Scale Prevention
Tech Manual Excerpt Water Chemistry and Pretreatment Strontium Sulfate Scale Prevention Strontium Calculation /8/ Sulfate Scale Prediction of SrSO scaling potential is performed in the same way as the previously Prevention 4 described procedure for CaSO4: 1. Calculate the ionic strength of the concentrate stream (Ic) following the procedure described in Scaling Calculations (Form No. 45-D01551-en): Eq. 1 2. Calculate the ion product (IPc) for SrSO4 in the concentrate stream: Eq. 2 where: m 2+ 2+ ( Sr )f = M Sr in feed, mol/L m 2– 2– ( SO4 )f = M SO4 in feed, mol/L 3. Compare IPc for SrSO4 with the solubility product (Ksp) of SrSO4 at the ionic strength of the concentrate stream, Figure 1 in Calcium Fluoride Scale Prevention (Form No. 45-D01556-en). If IPc ≥ 0.8 Ksp, SrSO4 scaling can occur, and adjustment is required. Adjustments for SrSO4 Scale Control The adjustments discussed in Calcium Sulfate Scale Prevention (Form No. 45-D01553-en) for CaSO4 scale control apply for SrSO4 scale control as well. Page 1 of 2 Form No. 45-D01555-en, Rev. 4 April 2020 Excerpt from FilmTec™ Reverse Osmosis Membranes Technical Manual (Form No. 45-D01504-en), Chapter 2, "Water Chemistry and Pretreatment." Have a question? Contact us at: All information set forth herein is for informational purposes only. This information is general information and may differ from that based on actual conditions. Customer is responsible for determining whether products and the information in this document are www.dupont.com/water/contact-us appropriate for Customer's use and for ensuring that Customer's workplace and disposal practices are in compliance with applicable laws and other government enactments. -

Toxicological Profile for Barium and Barium Compounds
TOXICOLOGICAL PROFILE FOR BARIUM AND BARIUM COMPOUNDS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 2007 BARIUM AND BARIUM COMPOUNDS ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. BARIUM AND BARIUM COMPOUNDS iii UPDATE STATEMENT A Toxicological Profile for Barium and Barium Compounds, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 BARIUM AND BARIUM COMPOUNDS iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. -
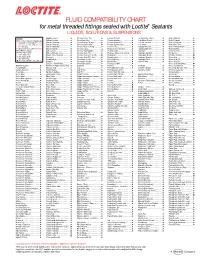
FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

C09c - 2020.01
CPC - C09C - 2020.01 C09C TREATMENT OF INORGANIC MATERIALS, OTHER THAN FIBROUS FILLERS, TO ENHANCE THEIR PIGMENTING OR FILLING PROPERTIES (preparation of inorganic compounds or non-metallic elements C01; treatment of materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone C04B 14/00, C04B 18/00, C04B 20/00); PREPARATION OF CARBON BLACK; {PREPARATION OF INORGANIC MATERIALS WHICH ARE NO SINGLE CHEMICAL COMPOUNDS AND WHICH ARE MAINLY USED AS PIGMENTS OR FILLERS} Definition statement This place covers: The treatment of inorganic compounds other than fibrous fillers, to enhance their pigmenting or filling properties; Preparation of carbon black. Preparation of inorganic materials being no single chemical compounds and used as pigments or fillers. References Limiting references This place does not cover: Treatment by polymerisation onto particle is classified in C08F 292/00. Only treatment by already polymerised agents is classified in C09C. Special rules of classification The following IPC groups are not used in the internal CPC classification scheme. Subject matter covered by these groups is classified in the following CPC groups: IPC group C09C1/68 is covered by CPC group C09K 3/14. Whenever in groups C09C 1/00 - C09C 1/66 the materials consist of a particulate core bearing a coating or any other deposit, classification is done only according to the composition of the core, unless otherwise stated, e.g. C09C 1/0015, C09C 1/0078. Preparations of materials which are no single chemical compounds, mainly comprising ceramic pigments (C09C 1/0009),consisting of solid solutions or polycristalline structures, and compounds defined as composite materials (C09C 1/0081).Preparation and treatment steps are not always easy to distinguish from each other, e.g.