Distribution and Evolution of Peroxisomes in Alveolates (Apicomplexa, Dinoflagellates, Ciliates)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Phylogenetic Analysis in Hammondia-Like Organisms Based on Partial Hsp70 Coding Sequences
1195 Molecular phylogenetic analysis in Hammondia-like organisms based on partial Hsp70 coding sequences R. M. MONTEIRO1, L. J. RICHTZENHAIN1,H.F.J.PENA1,S.L.P.SOUZA1, M. R. FUNADA1, S. M. GENNARI1, J. P. DUBEY2, C. SREEKUMAR2,L.B.KEID1 and R. M. SOARES1* 1 Departamento de Medicina Veterina´ria Preventiva e Sau´de Animal, Faculdade de Medicina Veterina´ria e Zootecnia, Universidade de Sa˜o Paulo, Av. Prof. Dr. Orlando Marques de Paiva, 87, CEP 05508-900, Sa˜o Paulo, SP, Brazil 2 Animal Parasitic Diseases Laboratory, Animal and Natural Resources Institute, Agricultural Research Service, United States Department of Agricultural, Building 1001, Beltsville, MD 20705, USA (Resubmitted 7 January 2007; revised 31 January 2007; accepted 5 February 2007; first published online 27 April 2007) SUMMARY The 70 kDa heat-shock protein (Hsp70) sequences are considered one of the most conserved proteins in all domains of life from Archaea to eukaryotes. Hammondia heydorni, H. hammondi, Toxoplasma gondii, Neospora hughesi and N. caninum (Hammondia-like organisms) are closely related tissue cyst-forming coccidians that belong to the subfamily Toxoplasmatinae. The phylogenetic reconstruction using cytoplasmic Hsp70 coding genes of Hammondia-like organisms revealed the genetic sequences of T. gondii, Neospora spp. and H. heydorni to possess similar levels of evolutionary distance. In addition, at least 2 distinct genetic groups could be recognized among the H. heydorni isolates. Such results are in agreement with those obtained with internal transcribed spacer-1 rDNA (ITS-1) sequences. In order to compare the nucleotide diversity among different taxonomic levels within Apicomplexa, Hsp70 coding sequences of the following apicomplexan organisms were included in this study: Cryptosporidium, Theileria, Babesia, Plasmodium and Cyclospora. -

The Transcriptome of the Avian Malaria Parasite Plasmodium
bioRxiv preprint doi: https://doi.org/10.1101/072454; this version posted August 31, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The Transcriptome of the Avian Malaria Parasite 2 Plasmodium ashfordi Displays Host-Specific Gene 3 Expression 4 5 6 7 8 Running title 9 The Transcriptome of Plasmodium ashfordi 10 11 Authors 12 Elin Videvall1, Charlie K. Cornwallis1, Dag Ahrén1,3, Vaidas Palinauskas2, Gediminas Valkiūnas2, 13 Olof Hellgren1 14 15 Affiliation 16 1Department of Biology, Lund University, Lund, Sweden 17 2Institute of Ecology, Nature Research Centre, Vilnius, Lithuania 18 3National Bioinformatics Infrastructure Sweden (NBIS), Lund University, Lund, Sweden 19 20 Corresponding authors 21 Elin Videvall ([email protected]) 22 Olof Hellgren ([email protected]) 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/072454; this version posted August 31, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 25 Abstract 26 27 Malaria parasites (Plasmodium spp.) include some of the world’s most widespread and virulent 28 pathogens, infecting a wide array of vertebrates. Our knowledge of the molecular mechanisms these 29 parasites use to invade and exploit hosts other than mice and primates is, however, extremely limited. 30 How do Plasmodium adapt to individual hosts and to the immune response of hosts throughout an 31 infection? To better understand parasite plasticity, and identify genes that are conserved across the 32 phylogeny, it is imperative that we characterize transcriptome-wide gene expression from non-model 33 malaria parasites in multiple host individuals. -

University of Oklahoma
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By JOSHUA THOMAS COOPER Norman, Oklahoma 2017 MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION APPROVED FOR THE DEPARTMENT OF MICROBIOLOGY AND PLANT BIOLOGY BY ______________________________ Dr. Boris Wawrik, Chair ______________________________ Dr. J. Phil Gibson ______________________________ Dr. Anne K. Dunn ______________________________ Dr. John Paul Masly ______________________________ Dr. K. David Hambright ii © Copyright by JOSHUA THOMAS COOPER 2017 All Rights Reserved. iii Acknowledgments I would like to thank my two advisors Dr. Boris Wawrik and Dr. J. Phil Gibson for helping me become a better scientist and better educator. I would also like to thank my committee members Dr. Anne K. Dunn, Dr. K. David Hambright, and Dr. J.P. Masly for providing valuable inputs that lead me to carefully consider my research questions. I would also like to thank Dr. J.P. Masly for the opportunity to coauthor a book chapter on the speciation of diatoms. It is still such a privilege that you believed in me and my crazy diatom ideas to form a concise chapter in addition to learn your style of writing has been a benefit to my professional development. I’m also thankful for my first undergraduate research mentor, Dr. Miriam Steinitz-Kannan, now retired from Northern Kentucky University, who was the first to show the amazing wonders of pond scum. Who knew that studying diatoms and algae as an undergraduate would lead me all the way to a Ph.D. -

Multiyear Survey of Coccidia, Cryptosporidia, Microsporidia, Histomona, and Hematozoa in Wild Quail in the Rolling Plains Ecoregion of Texas and Oklahoma, USA
Journal of Eukaryotic Microbiology ISSN 1066-5234 ORIGINAL ARTICLE Multiyear Survey of Coccidia, Cryptosporidia, Microsporidia, Histomona, and Hematozoa in Wild Quail in the Rolling Plains Ecoregion of Texas and Oklahoma, USA Lixin Xianga,b, Fengguang Guob, Yonglan Yuc, Lacy S. Parsonb, Lloyd LaCosted, Anna Gibsone, Steve M. Presleye, Markus Petersonf, Thomas M. Craigb, Dale Rollinsd,f, Alan M. Fedynichg & Guan Zhub a College of Life Science, Zhejiang University, Hangzhou, Zhejiang 310058, China b Department of Veterinary Pathobiology, College of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, Texas 77843-4467, USA c College of Veterinary Medicine, China Agricultural University, Haidian District, Beijing 100193, China d Rolling Plains Quail Research Foundation, San Angelo, Texas 76901, USA e Institute of Environmental & Human Health, Texas Tech University, Lubbock, Texas 79416, USA f Department of Wildlife & Fisheries Sciences, Texas A&M University, College Station, Texas 77843-2258, USA g Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville, Kingsville, Texas 78363, USA Keywords ABSTRACT Cryptosporidium; molecular epidemiology; northern bobwhite (Colinus virginianus); pro- We developed nested PCR protocols and performed a multiyear survey on the tozoan parasites; scaled quail (Callipepla prevalence of several protozoan parasites in wild northern bobwhite (Colinus squamata). virginianus) and scaled quail (Callipepla squamata) in the Rolling Plains ecore- gion of Texas and Oklahoma (i.e. fecal pellets, bird intestines and blood Correspondence smears collected between 2010 and 2013). Coccidia, cryptosporidia, and G. Zhu, Department of Veterinary Pathobiol- microsporidia were detected in 46.2%, 11.7%, and 44.0% of the samples ogy, College of Veterinary Medicine & (n = 687), whereas histomona and hematozoa were undetected. -

Journal of Parasitology
Journal of Parasitology Eimeria taggarti n. sp., a Novel Coccidian (Apicomplexa: Eimeriorina) in the Prostate of an Antechinus flavipes --Manuscript Draft-- Manuscript Number: 17-111R1 Full Title: Eimeria taggarti n. sp., a Novel Coccidian (Apicomplexa: Eimeriorina) in the Prostate of an Antechinus flavipes Short Title: Eimeria taggarti n. sp. in Prostate of Antechinus flavipes Article Type: Regular Article Corresponding Author: Jemima Amery-Gale, BVSc(Hons), BAnSci, MVSc University of Melbourne Melbourne, Victoria AUSTRALIA Corresponding Author Secondary Information: Corresponding Author's Institution: University of Melbourne Corresponding Author's Secondary Institution: First Author: Jemima Amery-Gale, BVSc(Hons), BAnSci, MVSc First Author Secondary Information: Order of Authors: Jemima Amery-Gale, BVSc(Hons), BAnSci, MVSc Joanne Maree Devlin, BVSc(Hons), MVPHMgt, PhD Liliana Tatarczuch David Augustine Taggart David J Schultz Jenny A Charles Ian Beveridge Order of Authors Secondary Information: Abstract: A novel coccidian species was discovered in the prostate of an Antechinus flavipes (yellow-footed antechinus) in South Australia, during the period of post-mating male antechinus immunosuppression and mortality. This novel coccidian is unusual because it develops extra-intestinally and sporulates endogenously within the prostate gland of its mammalian host. Histological examination of prostatic tissue revealed dense aggregations of spherical and thin-walled tetrasporocystic, dizoic sporulated coccidian oocysts within tubular lumina, with unsporulated oocysts and gamogonic stages within the cytoplasm of glandular epithelial cells. This coccidian was observed occurring concurrently with dasyurid herpesvirus 1 infection of the antechinus' prostate. Eimeria- specific 18S small subunit ribosomal DNA PCR amplification was used to obtain a partial 18S rDNA nucleotide sequence from the antechinus coccidian. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

Control of Intestinal Protozoa in Dogs and Cats
Control of Intestinal Protozoa 6 in Dogs and Cats ESCCAP Guideline 06 Second Edition – February 2018 1 ESCCAP Malvern Hills Science Park, Geraldine Road, Malvern, Worcestershire, WR14 3SZ, United Kingdom First Edition Published by ESCCAP in August 2011 Second Edition Published in February 2018 © ESCCAP 2018 All rights reserved This publication is made available subject to the condition that any redistribution or reproduction of part or all of the contents in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise is with the prior written permission of ESCCAP. This publication may only be distributed in the covers in which it is first published unless with the prior written permission of ESCCAP. A catalogue record for this publication is available from the British Library. ISBN: 978-1-907259-53-1 2 TABLE OF CONTENTS INTRODUCTION 4 1: CONSIDERATION OF PET HEALTH AND LIFESTYLE FACTORS 5 2: LIFELONG CONTROL OF MAJOR INTESTINAL PROTOZOA 6 2.1 Giardia duodenalis 6 2.2 Feline Tritrichomonas foetus (syn. T. blagburni) 8 2.3 Cystoisospora (syn. Isospora) spp. 9 2.4 Cryptosporidium spp. 11 2.5 Toxoplasma gondii 12 2.6 Neospora caninum 14 2.7 Hammondia spp. 16 2.8 Sarcocystis spp. 17 3: ENVIRONMENTAL CONTROL OF PARASITE TRANSMISSION 18 4: OWNER CONSIDERATIONS IN PREVENTING ZOONOTIC DISEASES 19 5: STAFF, PET OWNER AND COMMUNITY EDUCATION 19 APPENDIX 1 – BACKGROUND 20 APPENDIX 2 – GLOSSARY 21 FIGURES Figure 1: Toxoplasma gondii life cycle 12 Figure 2: Neospora caninum life cycle 14 TABLES Table 1: Characteristics of apicomplexan oocysts found in the faeces of dogs and cats 10 Control of Intestinal Protozoa 6 in Dogs and Cats ESCCAP Guideline 06 Second Edition – February 2018 3 INTRODUCTION A wide range of intestinal protozoa commonly infect dogs and cats throughout Europe; with a few exceptions there seem to be no limitations in geographical distribution. -

Characterization of Aminoacyl-Trna Synthetases in Chromerids
Article Characterization of Aminoacyl-tRNA Synthetases in Chromerids Abdoallah Sharaf 1,2, Ansgar Gruber 1, Kateřina Jiroutová 1 and Miroslav Oborník 1,3,* 1 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 České Budějovice, Czech Republic 2 Genetics Department, Faculty of Agriculture, Ain Shams University, Cairo 11241, Egypt 3 Faculty of Science, University of South Bohemia, 370 05 České Budějovice, Czech Republic * Correspondence: [email protected] Received: 1 July 2019; Accepted: 28 July 2019; Published: 31 July 2019 Abstract: Aminoacyl-tRNA synthetases (AaRSs) are enzymes that catalyze the ligation of tRNAs to amino acids. There are AaRSs specific for each amino acid in the cell. Each cellular compartment in which translation takes place (the cytosol, mitochondria, and plastids in most cases), needs the full set of AaRSs; however, individual AaRSs can function in multiple compartments due to dual (or even multiple) targeting of nuclear- encoded proteins to various destinations in the cell. We searched the genomes of the chromerids, Chromera velia and Vitrella brassicaformis, for AaRS genes: 48 genes encoding AaRSs were identified in C. velia, while only 39 AaRS genes were found in V. brassicaformis. In the latter alga, ArgRS and GluRS were each encoded by a single gene occurring in a single copy; only PheRS was found in three genes, while the remaining AaRSs were encoded by two genes. In contrast, there were nine cases for which C. velia contained three genes of a given AaRS (45% of the AaRSs), all of them representing duplicated genes, except AsnRS and PheRS, which are more likely pseudoparalogs (acquired via horizontal or endosymbiotic gene transfer). -

Clinical Parasitology: a Practical Approach
168 CHAPTER 7 Miscellaneous Protozoa proper personal hygiene, adequate sanitation known as Sarcocystis hominis. Similarly, Sarco- practices, and avoidance of unprotected sex, par- cystis suihominis may be found in pigs. In addi- ticularly among homosexual men. tion to these typical farm animals, a variety of wild animals may also harbor members of the Sarcocystis group. Sarcocystis lindemanni Quick Quiz! 7-5 has been designated as the umbrella term for those organisms that may potentially parasitize All the following are highly recommended when pro- humans. cessing samples for the identification of Isospora belli to ensure identification except: (Objective 7-8) A. Iodine wet prep Morphology B. Decreased microscope light level Mature Oocysts. Members of the genus Sar- C. Modified acid-fast stain cocystis were originally classified and considered D. Saline wet prep as members of the genus Isospora, in part because of the striking morphologic similarities of these parasites (Fig. 7-8; Table 7-4). The oval transpar- Quick Quiz! 7-6 ent organism consists of two mature sporocysts that each average from 10 to 18 μm in length. Which stage of reproduction is considered capable of Each sporocyst is equipped with four sausage- initiating another infection of Isospora belli? (Objec- shaped sporozoites. A double-layered clear and tives 7-5) colorless cell wall surrounds the sporocysts. A. Sporozoites B. Immature oocysts Laboratory Diagnosis C. Merozoites D. Mature oocysts Stool is the specimen of choice for the recovery of Sarcocystis organisms. The oocysts are usually passed into the feces fully developed. When present, these mature oocysts are typically seen Quick Quiz! 7-7 Which of the following patients would be more likely to contract an infection with Isospora belli? (Objective 7-6) Double layered A. -
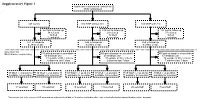
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Why the –Omic Future of Apicomplexa Should Include Gregarines Julie Boisard, Isabelle Florent
Why the –omic future of Apicomplexa should include Gregarines Julie Boisard, Isabelle Florent To cite this version: Julie Boisard, Isabelle Florent. Why the –omic future of Apicomplexa should include Gregarines. Biology of the Cell, Wiley, 2020, 10.1111/boc.202000006. hal-02553206 HAL Id: hal-02553206 https://hal.archives-ouvertes.fr/hal-02553206 Submitted on 24 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Article title: Why the –omic future of Apicomplexa should include Gregarines. Names of authors: Julie BOISARD1,2 and Isabelle FLORENT1 Authors affiliations: 1. Molécules de Communication et Adaptation des Microorganismes (MCAM, UMR 7245), Département Adaptations du Vivant (AVIV), Muséum National d’Histoire Naturelle, CNRS, CP52, 57 rue Cuvier 75231 Paris Cedex 05, France. 2. Structure et instabilité des génomes (STRING UMR 7196 CNRS / INSERM U1154), Département Adaptations du vivant (AVIV), Muséum National d'Histoire Naturelle, CP 26, 57 rue Cuvier 75231 Paris Cedex 05, France. Short Title: Gregarines –omics for Apicomplexa studies -

Enteric Protozoa in the Developed World: a Public Health Perspective
Enteric Protozoa in the Developed World: a Public Health Perspective Stephanie M. Fletcher,a Damien Stark,b,c John Harkness,b,c and John Ellisa,b The ithree Institute, University of Technology Sydney, Sydney, NSW, Australiaa; School of Medical and Molecular Biosciences, University of Technology Sydney, Sydney, NSW, Australiab; and St. Vincent’s Hospital, Sydney, Division of Microbiology, SydPath, Darlinghurst, NSW, Australiac INTRODUCTION ............................................................................................................................................420 Distribution in Developed Countries .....................................................................................................................421 EPIDEMIOLOGY, DIAGNOSIS, AND TREATMENT ..........................................................................................................421 Cryptosporidium Species..................................................................................................................................421 Dientamoeba fragilis ......................................................................................................................................427 Entamoeba Species.......................................................................................................................................427 Giardia intestinalis.........................................................................................................................................429 Cyclospora cayetanensis...................................................................................................................................430