Characterization of Aminoacyl-Trna Synthetases in Chromerids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review Article Diversity of Eukaryotic Translational Initiation Factor Eif4e in Protists
Hindawi Publishing Corporation Comparative and Functional Genomics Volume 2012, Article ID 134839, 21 pages doi:10.1155/2012/134839 Review Article Diversity of Eukaryotic Translational Initiation Factor eIF4E in Protists Rosemary Jagus,1 Tsvetan R. Bachvaroff,2 Bhavesh Joshi,3 and Allen R. Place1 1 Institute of Marine and Environmental Technology, University of Maryland Center for Environmental Science, 701 E. Pratt Street, Baltimore, MD 21202, USA 2 Smithsonian Environmental Research Center, 647 Contees Wharf Road, Edgewater, MD 21037, USA 3 BridgePath Scientific, 4841 International Boulevard, Suite 105, Frederick, MD 21703, USA Correspondence should be addressed to Rosemary Jagus, [email protected] Received 26 January 2012; Accepted 9 April 2012 Academic Editor: Thomas Preiss Copyright © 2012 Rosemary Jagus et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The greatest diversity of eukaryotic species is within the microbial eukaryotes, the protists, with plants and fungi/metazoa representing just two of the estimated seventy five lineages of eukaryotes. Protists are a diverse group characterized by unusual genome features and a wide range of genome sizes from 8.2 Mb in the apicomplexan parasite Babesia bovis to 112,000-220,050 Mb in the dinoflagellate Prorocentrum micans. Protists possess numerous cellular, molecular and biochemical traits not observed in “text-book” model organisms. These features challenge some of the concepts and assumptions about the regulation of gene expression in eukaryotes. Like multicellular eukaryotes, many protists encode multiple eIF4Es, but few functional studies have been undertaken except in parasitic species. -
![Downloaded from the Uni- [76] and Kept Only the Best Match with the Delta-Filter Protkb [85] Databank (9/2014) Were Aligned to the Gen- Command](https://docslib.b-cdn.net/cover/8007/downloaded-from-the-uni-76-and-kept-only-the-best-match-with-the-delta-filter-protkb-85-databank-9-2014-were-aligned-to-the-gen-command-938007.webp)
Downloaded from the Uni- [76] and Kept Only the Best Match with the Delta-Filter Protkb [85] Databank (9/2014) Were Aligned to the Gen- Command
Farhat et al. BMC Biology (2021) 19:1 https://doi.org/10.1186/s12915-020-00927-9 RESEARCH ARTICLE Open Access Rapid protein evolution, organellar reductions, and invasive intronic elements in the marine aerobic parasite dinoflagellate Amoebophrya spp Sarah Farhat1,2† , Phuong Le,3,4† , Ehsan Kayal5† , Benjamin Noel1† , Estelle Bigeard6, Erwan Corre5 , Florian Maumus7, Isabelle Florent8 , Adriana Alberti1, Jean-Marc Aury1, Tristan Barbeyron9, Ruibo Cai6, Corinne Da Silva1, Benjamin Istace1, Karine Labadie1, Dominique Marie6, Jonathan Mercier1, Tsinda Rukwavu1, Jeremy Szymczak5,6, Thierry Tonon10 , Catharina Alves-de-Souza11, Pierre Rouzé3,4, Yves Van de Peer3,4,12, Patrick Wincker1, Stephane Rombauts3,4, Betina M. Porcel1* and Laure Guillou6* Abstract Background: Dinoflagellates are aquatic protists particularly widespread in the oceans worldwide. Some are responsible for toxic blooms while others live in symbiotic relationships, either as mutualistic symbionts in corals or as parasites infecting other protists and animals. Dinoflagellates harbor atypically large genomes (~ 3 to 250 Gb), with gene organization and gene expression patterns very different from closely related apicomplexan parasites. Here we sequenced and analyzed the genomes of two early-diverging and co-occurring parasitic dinoflagellate Amoebophrya strains, to shed light on the emergence of such atypical genomic features, dinoflagellate evolution, and host specialization. Results: We sequenced, assembled, and annotated high-quality genomes for two Amoebophrya strains (A25 and A120), using a combination of Illumina paired-end short-read and Oxford Nanopore Technology (ONT) MinION long-read sequencing approaches. We found a small number of transposable elements, along with short introns and intergenic regions, and a limited number of gene families, together contribute to the compactness of the Amoebophrya genomes, a feature potentially linked with parasitism. -

Oborník M.& Lukeš, J. (2013) Cell Biology of Chromerids: Autotrophic
CHAPTER EIGHT Cell Biology of Chromerids: Autotrophic Relatives to Apicomplexan Parasites Miroslav Oborník*,†,{,1, Julius Lukeš*,† *Biology Centre, Institute of Parasitology, Academy of Sciences of the Czech Republic, Cˇ eske´ Budeˇjovice, Czech Republic †Faculty of Science, University of South Bohemia, Cˇ eske´ Budeˇjovice, Czech Republic { Institute of Microbiology, Academy of Sciences of the Czech Republic, Trˇebonˇ, Czech Republic 1Corresponding author: e-mail address: [email protected] Contents 1. Introduction 334 2. Chromerida: A New Group of Algae Isolated from Australian Corals 337 2.1 C. velia: A new alga from Sydney Harbor 338 2.2 V. brassicaformis: An alga from the Great Barrier Reef 343 3. Life Cycle 346 4. Evolution of Exosymbiont 348 5. Evolution of Chromerid Organelles 350 5.1 Evolution of chromerid plastids 350 5.2 Reduced mitochondrial genomes of chromerids 354 5.3 Chromerosome: C. velia as a possible mixotroph 354 6. Metabolism of Chromerids 355 6.1 Unique pathway for tetrapyrrole biosynthesis 355 6.2 Other metabolic features of C. velia 359 7. Chromerids as Possible Symbionts of Corals 361 8. Conclusions 361 Acknowledgments 362 References 362 Abstract Chromerida are algae possessing a complex plastid surrounded by four membranes. Although isolated originally from stony corals in Australia, they seem to be globally dis- tributed. According to their molecular phylogeny, morphology, ultrastructure, structure of organellar genomes, and noncanonical pathway for tetrapyrrole synthesis, these algae are thought to be the closest known phototrophic relatives to apicomplexan par- asites. Here, we summarize the current knowledge of cell biology and evolution of this novel group of algae, which contains only two formally described species, but is appar- ently highly diverse and virtually ubiquitous in marine environments. -

(PAD) and Post-Translational Protein Deimination—Novel Insights Into Alveolata Metabolism, Epigenetic Regulation and Host–Pathogen Interactions
biology Article Peptidylarginine Deiminase (PAD) and Post-Translational Protein Deimination—Novel Insights into Alveolata Metabolism, Epigenetic Regulation and Host–Pathogen Interactions Árni Kristmundsson 1,*, Ásthildur Erlingsdóttir 1 and Sigrun Lange 2,* 1 Institute for Experimental Pathology at Keldur, University of Iceland, Keldnavegur 3, 112 Reykjavik, Iceland; [email protected] 2 Tissue Architecture and Regeneration Research Group, School of Life Sciences, University of Westminster, London W1W 6UW, UK * Correspondence: [email protected] (Á.K.); [email protected] (S.L.) Simple Summary: Alveolates are a major group of free living and parasitic organisms; some of which are serious pathogens of animals and humans. Apicomplexans and chromerids are two phyla belonging to the alveolates. Apicomplexans are obligate intracellular parasites; that cannot complete their life cycle without exploiting a suitable host. Chromerids are mostly photoautotrophs as they can obtain energy from sunlight; and are considered ancestors of the apicomplexans. The pathogenicity and life cycle strategies differ significantly between parasitic alveolates; with some causing major losses in host populations while others seem harmless to the host. As the life cycles of Citation: Kristmundsson, Á.; Erlingsdóttir, Á.; Lange, S. some are still poorly understood, a better understanding of the factors which can affect the parasitic Peptidylarginine Deiminase (PAD) alveolates’ life cycles and survival is of great importance and may aid in new biomarker discovery. and Post-Translational Protein This study assessed new mechanisms relating to changes in protein structure and function (so-called Deimination—Novel Insights into “deimination” or “citrullination”) in two key parasites—an apicomplexan and a chromerid—to Alveolata Metabolism, Epigenetic assess the pathways affected by this protein modification. -

The Organellar Genomes of Chromera and Vitrella, the Phototrophic
MI69CH07-Lukes ARI 5 June 2015 13:47 V I E E W R S I E N C N A D V A The Organellar Genomes of Chromera and Vitrella,the Phototrophic Relatives of Apicomplexan Parasites Miroslav Obornık´ 1,2,3 and Julius Lukesˇ1,2,4 1Institute of Parasitology, Biology Center, Czech Academy of Sciences, 1160/31 Ceskˇ e´ Budejovice,ˇ Czech Republic; email: [email protected], [email protected] 2Faculty of Science, University of South Bohemia, 37005 Ceskˇ e´ Budejovice,ˇ Czech Republic 3Institute of Microbiology, Czech Academy of Sciences, 379 81 Treboˇ n,ˇ Czech Republic 4Canadian Institute for Advanced Research, Toronto, Ontario M5G 1Z8, Canada Annu. Rev. Microbiol. 2015. 69:129–44 Keywords The Annual Review of Microbiology is online at organellar genomes, mitochondrion, plastid, Apicomplexa, Alveolata, micro.annualreviews.org Chromera This article’s doi: 10.1146/annurev-micro-091014-104449 Abstract Copyright c 2015 by Annual Reviews. Apicomplexa are known to contain greatly reduced organellar genomes. All rights reserved Their mitochondrial genome carries only three protein-coding genes, and their plastid genome is reduced to a 35-kb-long circle. The discovery of coral- endosymbiotic algae Chromera velia and Vitrella brassicaformis, which share a common ancestry with Apicomplexa, provided an opportunity to study possibly ancestral forms of organellar genomes, a unique glimpse into the evolutionary history of apicomplexan parasites. The structurally similar mi- tochondrial genomes of Chromera and Vitrella differ in gene content, which is reflected in the composition of their respiratory chains. Thus, Chromera lacks respiratory complexes I and III, whereas Vitrella and apicomplexan parasites are missing only complex I. -

Light Harvesting Complexes of Chromera Velia, Photosynthetic Relative of Apicomplexan Parasites
Biochimica et Biophysica Acta 1827 (2013) 723–729 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbabio Light harvesting complexes of Chromera velia, photosynthetic relative of apicomplexan parasites Josef Tichy a,b, Zdenko Gardian a,b, David Bina a,b, Peter Konik a, Radek Litvin a,b, Miroslava Herbstova a,b, Arnab Pain c, Frantisek Vacha a,b,⁎ a Faculty of Science, University of South Bohemia, Branisovska 31, 37005 Ceske Budejovice, Czech Republic b Institute of Plant Molecular Biology, Biology Centre ASCR, Branisovska 31, 37005 Ceske Budejovice, Czech Republic c Computational Bioscience Research Center, King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia article info abstract Article history: The structure and composition of the light harvesting complexes from the unicellular alga Chromera velia Received 19 October 2012 were studied by means of optical spectroscopy, biochemical and electron microscopy methods. Two different Received in revised form 31 January 2013 types of antennae systems were identified. One exhibited a molecular weight (18–19 kDa) similar to FCP Accepted 5 February 2013 (fucoxanthin chlorophyll protein) complexes from diatoms, however, single particle analysis and circular Available online 18 February 2013 dichroism spectroscopy indicated similarity of this structure to the recently characterized XLH antenna of “ ” Keywords: xanthophytes. In light of these data we denote this antenna complex CLH, for Chromera Light Harvesting fi Chromera velia complex. The other system was identi ed as the photosystem I with bound Light Harvesting Complexes FCP (PSI–LHCr) related to the red algae LHCI antennae. The result of this study is the finding that C. -

CURRICULUM VITAE Saddef Haq, Ph.D. Program in Toxicology University of Maryland Baltimore School of Medicine
Beyond the Dinoflagellate Transcriptome: Validation of Protein Production via Biochemical Analysis and Mass Spectrometry Item Type dissertation Authors Haq, Saddef Publication Date 2018 Abstract Dinoflagellates are members of the Alveolata (meaning “with cavities”), a monophyletic group of single cell protists which includes apicomplexans and ciliates that exhibit a diverse mode of nutrition, ranging from predation to photo autotrophy to int... Keywords bioluminescence; dinoflagellates; toxin synthesis; Acetyl-CoA Carboxylase; Dinoflagellida; Polyketide Synthases; Proteomics Download date 27/09/2021 16:23:13 Link to Item http://hdl.handle.net/10713/8970 CURRICULUM VITAE Saddef Haq, Ph.D. Program in Toxicology University of Maryland Baltimore School of Medicine Institute of Marine and Environmental Technology 701 E. Pratt Street Baltimore, Maryland 21202 Email: [email protected] EDUCATION 2013-2018 University of Maryland, Baltimore, MD Program in Toxicology Doctor of Philosophy 2006-2008 Rutgers University, New Brunswick, NJ Bachelor of Science in Biochemistry 2003-2006 Union County College, Cranford, NJ Associates of Science in Biology EMPLOYMENT HISTORY July 2013-Present Graduate Research Assistant Graduate Program in Life Sciences, Program in Toxicology University of Maryland, Baltimore, Baltimore, MD Lab of Dr. Allen R. Place at the Institute of Marine and Environmental Technology, Baltimore, MD. Sep 2010-May 2013 Senior Research Technician Center for Infection and Immunity Columbia University, School of Public Health New York, NY -
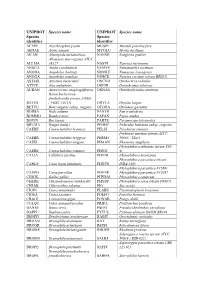
Additional File 1 Revised.Pdf
UNIPROT Species name UNIPROT Species name Species Species identifier identifier ACYPI Acyrthosiphon pisum MUSPF Mustela putorius furo AEDAE Aedes aegypti MYOLU Myotis lucifugus AILME Ailuropoda melanoleuca NAEGR Naegleria gruberi Allomyces macrogynus ATCC ALLMA 38327 NASVI Nasonia vitripennis ANOCA Anolis carolinensis NEMVE Nematostella vectensis ANODA Anopheles darlingi NOMLE Nomascus leucogenys ANOGA Anopheles gambiae NOSCE Nosema ceranae (strain BRL01) ASTMX Astyanax mexicanus ONCVO Onchocerca volvulus ATTCE Atta cephalotes ORENI Oreochromis niloticus AURAN Aureococcus anophagefferens ORNAN Ornithorhynchus anatinus Batrachochytrium dendrobatidis (strain JAM81 BATDJ / FGSC 10211) ORYLA Oryzias latipes BETVU Beta vulgaris subsp. vulgaris OTOGA Otolemur garnettii BODSA Bodo saltans PANTR Pan troglodytes BOMMO Bombyx mori PAPAN Papio anubis BOVIN Bos taurus PARTE Paramecium tetraurelia BRUMA Brugia malayi PEDHC Pediculus humanus subsp. corporis CAEBE Caenorhabditis brenneri PELSI Pelodiscus sinensis Perkinsus marinus (strain ATCC CAEBR Caenorhabditis briggsae PERM5 50983 / TXsc) CAEEL Caenorhabditis elegans PHAAN Phaseolus angularis Phytophthora infestans (strain T30- CAERE Caenorhabditis remanei PHYIT 4) CALJA Callithrix jacchus PHYNI Phytophthora nicotianae Phytophthora parasitica (strain CANLF Canis lupus familiaris PHYPN INRA-310) Phytophthora parasitica P1569/ CAVPO Cavia porcellus PHYPR Phytophthora parasitica P10297 CHICK Gallus gallus PHYRM Phytophthora ramorum CHLRE Chlamydomonas reinhardtii PHYSP Phytophthora sojae (strain P6497) -

Infection of Ceratium Furca by the Parasitic Dinoflagellate Amoebophrya Ceratii (Amoebophryidae) in the Mexican Pacific
Acta Botanica Mexicana (2003), 65: 1-9 INFECTION OF CERATIUM FURCA BY THE PARASITIC DINOFLAGELLATE AMOEBOPHRYA CERATII (AMOEBOPHRYIDAE) IN THE MEXICAN PACIFIC ISMAEL GÁRATE LIZÁRRAGA Laboratorio de Fitoplancton, Departamento de Plancton y Ecología Marina, Centro Interdisciplinario de Ciencias Marinas-I.P.N. Apartado Postal 592; 23000 La Paz, Baja California Sur, México DAVID A. SIQUEIROS BELTRONES Departamento de Biología Marina, Universidad Autónoma de Baja California Sur Apartado Postal 19-B; 23081 La Paz, Baja California Sur, México y Laboratorio de Fitoplancton, Departamento de Plancton y Ecología Marina, Centro Interdisciplinario de Ciencias Marinas-I.P.N., La Paz, Baja California Sur ABSTRACT Parasitism within dinoflagellates is a widespread and well-documented phenomenon. Parasitic dinoflagellates of the genus Amoebophrya commonly infect free-living toxic, and non- toxic dinoflagellates species which may cause harmful red tides. Infections of Ceratium furca by A. ceratii were observed in red tides samples collected in the northwest coast of Baja California between 30°01'05'' N, 115°51'16'' W and 31°09'33'' N, 116°31'09'' W. This is the first record of this particular parasitic dinoflagellate in Mexican Pacific waters. There were mainly three dinoflagellate species causing this particular seawater discoloration: a Gymnodinium- like dinoflagellate, Ceratium furca, and Akashiwo sanguinea. These reached concentrations as high as 560 000, 762 600, and 395 400 cells L-1, respectively. During the bloom, surface water temperature ranged between 13 and 17°C. Seawater salinity ranged from 33.2 to 33.8 psu. About 1.5% of the individuals of C. furca observed were infected by the intracellular parasite dinoflagellate Amoebophrya ceratii. -

Infection by Amoebophrya Spp. Parasitoids of Dinoflagellates in a Tropical Marine Coastal Area
Vol. 55: 143–153, 2009 AQUATIC MICROBIAL ECOLOGY Published online April 23, 2009 doi: 10.3354/ame01293 Aquat Microb Ecol Infection by Amoebophrya spp. parasitoids of dinoflagellates in a tropical marine coastal area Paulo S. Salomon1,*, Edna Granéli1, Maria H. C. B. Neves2, Eliane G. Rodriguez2 1Marine Sciences Centre, University of Kalmar, 39182 Kalmar, Sweden 2Instituto de Estudos do Mar Almirante Paulo Moreira, Rua Kioto 253, 28930-000 Arraial do Cabo, Rio de Janeiro, Brazil ABSTRACT: Infection of marine dinoflagellates by the parasitic dinoflagellate Amoebophrya spp. plays an important role in population dynamics and carbon flow in marine food webs. It has been extensively reported that Amoebophrya parasitoids occur in temperate coastal areas of the northern hemisphere; however, little is known about their distribution and importance in tropical areas and southern oceans. We used an rRNA-based, fluorescent in situ hybridization assay to detect Amoe- bophrya spp. infections during the decline of a late-summer dinoflagellate population dominated by Ceratium falcatiforme in a tropical coastal area of the southern Atlantic Ocean subjected to recurrent upwelling–downwelling cycles. Conditions during our survey were typical of downwelling when oligotrophic waters dominate the area. C. falcatiforme was the most infected host, with a prevalence averaging 2% over the study area at the beginning of sampling. At a fixed sampling station moni- tored over 4 wk, Amoebophrya prevalence escalated from 1 to 7% over a 6 d period, concomitant to a 94% decrease in host cell numbers. Infection by Amoebophrya was estimated to have killed ca. 11% of the host cell population within this period; thus, parasitism was not the main factor behind the C. -

Phylogenetics of Cladopyxidoiddinophytes
www.nature.com/scientificreports OPEN Fensomea setacea, gen. & sp. nov. (Cladopyxidaceae, Dinophyceae), is neither gonyaulacoid nor peridinioid as inferred from morphological and molecular data Marc Gottschling1, Maria Consuelo Carbonell‑Moore2, Kenneth Neil Mertens3, Monika Kirsch4, Malte Elbrächter5 & Urban Tillmann6* Dinophyte evolution is essentially inferred from the pattern of thecal plates, and two diferent labelling systems are used for the important subgroups Gonyaulacales and Peridiniales. The partiform hypotheca of cladopyxidoid dinophytes fts into the morphological concepts of neither group, although they are assigned to the Gonyaulacales. Here, we describe the thecate dinophyte Fensomea setacea, gen. & sp. nov., which has a cladopyxidoid tabulation. The cells displayed a Kofoidean plate formula APC, 3′, 4a, 7″, 7C, 6S, 6′′′, 2′′′′, and slender processes were randomly distributed over the echinate or baculate surface. In addition, we obtained rRNA sequences of F. setacea, gen. & sp. nov., but dinophytes that exhibit a partiform hypotheca did not show a close relationship to Gonyaulacales. Character evolution of thecate dinophytes may have progressed from the ancestral state of six postcingular plates, and two more or less symmetrically arranged antapical plates, towards patterns of only fve postcingular plates (Peridiniales) or more asymmetrical confgurations (Gonyaulacales). Based on our phylogenetic reconsiderations the contact between the posterior sulcal plate and the frst postcingular plate, as well as the contact between an antapical plate and the distalmost postcingular plate, do not represent a rare, specialized gonyaulacoid plate confguration (i.e., the partiform hypotheca of cladopyxidoid dinophytes). Instead, these contacts correspond to the common and regular confguration of peridinioid (and other) dinophytes. Over time, evolution has produced impressive biodiversity in the world’s oceans, including multicellular organ- isms, such as animals, and unicellular organisms that make signifcant ecological contributions 1,2. -

A Method for Identification of Highly Conserved Elements and Evolutionary Analysis of Superphylum Alveolata Lev I
Rubanov et al. BMC Bioinformatics (2016) 17:385 DOI 10.1186/s12859-016-1257-5 RESEARCH ARTICLE Open Access A method for identification of highly conserved elements and evolutionary analysis of superphylum Alveolata Lev I. Rubanov*, Alexandr V. Seliverstov, Oleg A. Zverkov and Vassily A. Lyubetsky Abstract Background: Perfectly or highly conserved DNA elements were found in vertebrates, invertebrates, and plants by various methods. However, little is known about such elements in protists. The evolutionary distance between apicomplexans can be very high, in particular, due to the positive selection pressure on them. This complicates the identification of highly conserved elements in alveolates, which is overcome by the proposed algorithm. Results: A novel algorithm is developed to identify highly conserved DNA elements. It is based on the identification of dense subgraphs in a specially built multipartite graph (whose parts correspond to genomes). Specifically, the algorithm does not rely on genome alignments, nor pre-identified perfectly conserved elements; instead, it performs a fast search for pairs of words (in different genomes) of maximum length with the difference below the specified edit distance. Such pair defines an edge whose weight equals the maximum (or total) length of words assigned to its ends. The graph composed of these edges is then compacted by merging some of its edges and vertices. The dense subgraphs are identified by a cellular automaton-like algorithm; each subgraph defines a cluster composed of similar inextensible words from different genomes. Almost all clusters are considered as predicted highly conserved elements. The algorithm is applied to the nuclear genomes of the superphylum Alveolata, and the corresponding phylogenetic tree is built and discussed.