Development and Gastrulation in Hyloxalus Vertebralis and Dendrobates Auratus (Anura: Dendrobatidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcription Factors Mix1 and Vegt, Relocalization of Vegt Mrna, and Conserved Endoderm and Dorsal Specification in Frogs
Transcription factors Mix1 and VegT, relocalization of vegt mRNA, and conserved endoderm and dorsal specification in frogs Norihiro Sudoua,b, Andrés Garcés-Vásconezc, María A. López-Latorrec, Masanori Tairaa, and Eugenia M. del Pinoc,1 aDepartment of Biological Sciences, Graduate School of Science, University of Tokyo, Tokyo 113-0033, Japan; bDepartment of Anatomy, School of Medicine, Tokyo Women’s Medical University, Tokyo 162-8666, Japan; and cEscuela de Ciencias Biológicas, Pontificia Universidad Católica del Ecuador, Quito 170517, Ecuador Contributed by Eugenia M. del Pino, April 6, 2016 (sent for review December 16, 2015; reviewed by Igor B. Dawid and Jean-Pierre Saint-Jeannet) Protein expression of the transcription factor genes mix1 and vegt the posterior mesoderm (13). mix1 encodes a paired-like home- characterized the presumptive endoderm in embryos of the frogs odomain transcription factor expressed ubiquitously in the endo- Engystomops randi, Epipedobates machalilla, Gastrotheca riobambae, derm and mesoderm (14). Its expression is detected in the and Eleutherodactylus coqui,asinXenopus laevis embryos. Protein mesendoderm shortly after the mid-blastula transition and ex- VegT was detected in the animal hemisphere of the early blastula in pression peaks in the presumptive endoderm and mesoderm of all frogs, and only the animal pole was VegT-negative. This finding the X. laevis gastrula (14, 15). mix1 has not been sequenced in stimulated a vegt mRNA analysis in X. laevis eggs and embryos. vegt frogs other than Xenopus/Silurana. mRNA was detected in the animal region of X. laevis eggs and early The vegt ORF has been sequenced in the amphibians E. coqui Ec_vegt Rana pipiens Rp_vegt Ambystoma mexicanum embryos, in agreement with the VegT localization observed in the ( ), ( ), and (Am_vegt)(16–18) and partially sequenced in Epipedobates analyzed frogs. -

Thermal Adaptation of Amphibians in Tropical Mountains
Thermal adaptation of amphibians in tropical mountains. Consequences of global warming Adaptaciones térmicas de anfibios en montañas tropicales: consecuencias del calentamiento global Adaptacions tèrmiques d'amfibis en muntanyes tropicals: conseqüències de l'escalfament global Pol Pintanel Costa ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. -

N.Orntates PUBLISHED by the AMERICAN MUSEUM of NATURAL HISTORY CENTRAL PARK WEST at 79TH STREET, NEW YORK, N.Y
AMERICAN MUSEUM N.orntates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 3068, 15 pp., 12 figures, 1 table June 11, 1993 A New Poison Frog from Manu INational Park, Southeastern Peru (Dendrobatidae, Epipedobates) LILY RODRIGUEZ' AND CHARLES W. MYERS2 ABSTRACT Epipedobates macero is a new species of den- ilar to a few other species occurring along the An- drobatid poison frog from lowland rain forest of dean front in eastern Peru, namely E. petersi and the Manu National Park, in the upper Madre de E. cainarachi, which differ in details ofcoloration, Dios drainage ofsoutheastern Peru. It is most sim- morphology, and vocalization. RESUMEN Epipedobates macero, especie nueva, es un den- del llano amaz6nico al pie de los Andes orientales drobatido venenoso de la selva pluvial baja del peruanos, a saber, E. petersi y E. cainarachi, las Parque Nacional del Manu, en el drenaje del Rio cuales difieren en detalles de coloracion, morfo- Alto Madre de Dios, al sudeste del Peru. Es similar logla, y vocalizacion. a otras dos especies que ocurren en los bosques ' Field Associate, Department of Herpetology and Ichthyology, American Museum of Natural History. Investi- gadora Asociada: Asociaci6n Peruana para la Conservaci6n de la Naturaleza (APECO), Parque Jos6 de Acosta 187, Lima 17, Perfi; and Museo de Historia Natural de la Universidad Mayor de San Marcos, apartado 140434, Lima 14, Peru. 2 Curator, Department of Herpetology and Ichthyology, American Museum of Natural History. Copyright © American Museum of Natural History 1993 ISSN 0003-0082 / Price $3.90 2 AMERICAN MUSEUM NOVITATES NO. -

Table 7: Species Changing IUCN Red List Status (2018-2019)
IUCN Red List version 2019-3: Table 7 Last Updated: 10 December 2019 Table 7: Species changing IUCN Red List Status (2018-2019) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2018 (IUCN Red List version 2018-2) and 2019 (IUCN Red List version 2019-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2018) List (2019) change version Category -
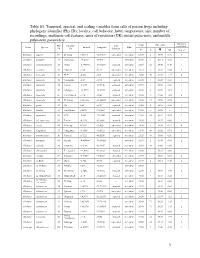
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance
Chapter 21 A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance Juan C. Santos , Rebecca D. Tarvin , and Lauren A. O’Connell 21.1 Introduction Chemical defense has evolved multiple times in nearly every major group of life, from snakes and insects to bacteria and plants (Mebs 2002 ). However, among land vertebrates, chemical defenses are restricted to a few monophyletic groups (i.e., clades). Most of these are amphibians and snakes, but a few rare origins (e.g., Pitohui birds) have stimulated research on acquired chemical defenses (Dumbacher et al. 1992 ). Selective pressures that lead to defense are usually associated with an organ- ism’s limited ability to escape predation or conspicuous behaviors and phenotypes that increase detectability by predators (e.g., diurnality or mating calls) (Speed and Ruxton 2005 ). Defended organisms frequently evolve warning signals to advertise their defense, a phenomenon known as aposematism (Mappes et al. 2005 ). Warning signals such as conspicuous coloration unambiguously inform predators that there will be a substantial cost if they proceed with attack or consumption of the defended prey (Mappes et al. 2005 ). However, aposematism is likely more complex than the simple pairing of signal and defense, encompassing a series of traits (i.e., the apose- matic syndrome) that alter morphology, physiology, and behavior (Mappes and J. C. Santos (*) Department of Zoology, Biodiversity Research Centre , University of British Columbia , #4200-6270 University Blvd , Vancouver , BC , Canada , V6T 1Z4 e-mail: [email protected] R. D. Tarvin University of Texas at Austin , 2415 Speedway Stop C0990 , Austin , TX 78712 , USA e-mail: [email protected] L. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Reproductive Biology of Ameerega Trivittata(Anura: Dendrobatidae)
ACTA AMAZONICA http://dx.doi.org/10.1590/1809-4392201305384 Reproductive biology of Ameerega trivittata (Anura: Dendrobatidae) in an area of terra firme forest in eastern Amazonia Ellen Cristina Serrão ACIOLI1 * , Selvino NECKEL-OLIVEIRA2 ¹ Universidade Federal do Pará/Museu Paraense Emílio Goeldi, Programa de Pós Graduação em Zoologia, Av. Perimetral 1901/1907, Terra Firme, 66017-970, Belém, Pará, Brasil. ² Universidade Federal de Santa Catarina, Departamento de Ecologia e Zoologia, Centro de Ciências Biológicas, 88040-970, Florianópolis, Santa Catarina, Brasil. * Corresponding author: [email protected] ABSTRACT The reproductive success of tropical amphibians is influenced by factors such as body size and the characteristics of breeding sites. Data on reproductive biology are important for the understanding of population dynamics and the maintenance of species. The objectives of the present study were to examine the abundance ofAmeerega trivittata, analyze the use of microhabitats by calling males and the snout-vent length (SVL) of breeding males and females, the number of tadpoles carried by the males and mature oocytes in the females, as well as the relationship between the SVL of the female and both the number and mean size of the mature oocytes found in the ovaries. Three field trips were conducted between January and September, 2009. A total of 31 plots, with a mean area of 2.3 ha, were surveyed, resulting in records of 235 individuals, with a mean density of 3.26 individuals per hectare. Overall, 66.1% of the individuals sighted were located in the leaf litter, while 17.4% were perched on decaying tree trunks on the forest floor, 15.7% on the aerial roots ofCecropia trees, and 0.8% on lianas. -

Summary Record of the 26Th Meeting of the Animals Committee
Original language: English AC26 summary record CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-sixth meeting of the Animals Committee Geneva (Switzerland), 15-20 March 2012 and Dublin (Ireland), 22-24 March 2012 SUMMARY RECORD Animals Committee matters 1. Opening of the meeting The Chair opened the meeting and welcomed all participants, before giving the floor to the Secretary- General, who also welcomed everyone and introduced new members of the Secretariat's scientific team (Mr De Meulenaer and Ms Kwitsinskaia) and enforcement team (Ms Garcia Ferreira, Ms Jonsson and Mr van Rensburg). He wished the Committee well in its deliberations. The Chair thanked the Secretary-General and invited suggestions as to how the Conference of the Parties could establish stronger measures to support the Committee as well as export countries, which deserved particular assistance. No other intervention was made during discussion of this item.1 2. Rules of Procedure The Secretariat introduced document AC26 Doc. 2 and proposed amending Rule 22 as follows: “On request, the Secretariat shall distribute printed and translated documents...”. The Secretariat explained that most members regularly indicated that they did not need printed copies and that this proposal was made to reduce costs. Although not opposed to the change in principle, a Party regretted that the suggestion had not been presented in the document, which would have given Parties time to consider it, and was concerned that this unannounced proposal might create a precedent. Another Party asked a question on the procedure to accept observers, but the Chair invited it to raise this topic under agenda item 4 on Admission of observers. -

De Los Anfibios Del Páramo
guía dinámica de los anfibios del páramo santiago ron coordinador editorial Lista de especies Número de especies: 39 Anura Hemiphractidae Gastrotheca espeletia, Rana marsupial de La Cocha Gastrotheca litonedis, Rana marsupial azuaya Gastrotheca pseustes, Rana marsupial de San Lucas Bufonidae Atelopus bomolochos, Jambato de Cuenca Atelopus exiguus, Jambato de Mazán Atelopus nanay, Jambato de las Tres Cruces Atelopus pastuso, Jambato pastuso Atelopus petersi, Jambato de Peters Atelopus podocarpus, Jambato de Podocarpus Atelopus ignescens, Jambato negro Osornophryne angel, Osornosapo del Ángel Osornophryne antisana, Osornosapo de Antisana Osornophryne talipes, Osornosapo trompudo Telmatobiidae Telmatobius niger, Uco de manchas naranjas Telmatobius vellardi, Uco de Vellard Hylidae Hyloscirtus larinopygion, Rana de torrente pastusa Strabomantidae Pristimantis devillei, Cutín de Ville Pristimantis leoni, Cutín de León Pristimantis pycnodermis, Cutín de antifaz Pristimantis festae, Cutín paramero Pristimantis buckleyi, Cutín de Imbabura Pristimantis cryophilius, Cutín de San Vicente Pristimantis curtipes, Cutín de Intac Pristimantis gentryi, Cutín de Pilalo Pristimantis modipeplus, Cutín de Urbina Pristimantis ocreatus, Cutín del Carchi Pristimantis orcesi, Cutín de Orcés Pristimantis orestes, Cutín de Urdaneta Pristimantis riveti, Cutín de Riveti Pristimantis unistrigatus, Cutín de Quito Pristimantis thymelensis, Cutín del paramo del Angel Pristimantis mazar, Cutín de Mazar Pristimantis philipi, Cutín de Philip Pristimantis gualacenio, Cutín -

TESIS DGBARRAGAN.Pdf
PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR FACULTAD DE CIENCIAS EXACTAS Y NATURALES ESCUELA DE CIENCIAS BIOLÓGICAS Effects of future climate change and habitat loss in the distribution of frog species in the Ecuadorian Andes Tesis previa a la obtención del título de Magíster en Biología de la Conservación DAYANA GABRIELA BARRAGÁN ALTAMIRANO Quito, 2015 iii Certifico que la tesis de Maestría en Biología de la Conservación de la candidata Dayana Gabriela Barragán Altamirano ha sido concluida de conformidad con las normas establecidas; por lo tanto, puede ser presentada para la calificación correspondiente. Firma de Director de Tesis Fecha: 27 diciembre de 2015 iv ACKNOWLEDGEMENTS This study has benefited from two valuable reviewers Veronica Crespo and Santiago Mena, who generously contributed with observations to improve this study. We also want to thank Santiago Burneo for guidance on the ecological niche modeling section, and Julio Sanchez, for his guidance on the section of statistical data analysis. We want to thank Damian Nicolalde for facilitating the data base of species occurrence records and Pablo Venegas for his assistance in taxonomic identification. We acknowledge Francisco Prieto, Danilo Granja, Janeth Delgado, Carlos Ponce (Ministerio del Ambiente), Alejandro Oviedo (Ministerio de Transporte y Obras Públicas), Pamela Hidalgo, and Francisco Moscoso who generously authorized and shared geographical information useful to perform the predictive habitat loss model. We want to thank Luis Camacho who helped to review the manuscript. Finally, Sean McCartney, Michelle Andrews (Clark Labs Technical Support), Santiago Silva (Centro de Recursos IDRISI Ecuador) offered their assistance during the predicting habitat loss modeling. v TABLE OF CONTENTS 1. -

October/November 2005
October/November 2005 Welcome to the second issue of Dartfrog ‘News’ Where does the time go? It seems like only yesterday we were sending out the maiden issue of this newsletter. So what has happened in between? Well, we went to the Bocas Del Toro archipelago in northeast Panama and to say that it was a dart frog paradise would be quite an understatement – it literally was dart frog heaven. In this issue we have the first instalment of our Bocas diary. We also have some photos of new dart frog acquisitions, of various tadpoles and a review of the excellent tree fern root or xaxim products. On the caudata front there are photos of egg/larvae development in the emperor salamander (Tylototriton shanjiing). As always there are other offers, some amazing frogs for sale that will not appear on our general web pricelist and tips and hints... “Amphibian of the month” Zaparo’s poison frog (Allobates (Epipedobates) zaparo) We first came across this species at one of the Dutch frog day shows near Amsterdam in October 2003. The specimens we picked up were rather small (about 10mm) and non- descript, just beginning to show the prominent yellow flash marks. Having never seen this species in true life we had no idea at the time what they would turn out like. We need not have worried – the dorsum gradually become more and more granular and an increasingly deep red in coloration. In the wild this species hails from Eastern Ecuador, northern Peru and possibly into southern Colombia and are concentrated around the humid forests of the Rio Pastaza and Rio Napo river basins.