Poison Frogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predation Upon Mantella Aurantiaca in the Torotorofotsy Wetlands, Central-Eastern Madagascar
Herpetology Notes, volume 2: 95-97 (2009) (published online on 10 July 2009) Predation upon Mantella aurantiaca in the Torotorofotsy wetlands, central-eastern Madagascar Olga Jovanovic1*, Miguel Vences1, Goran Safarek2, Falitiana C.E. Rabemananjara3, Rainer Dolch4 Abstract. Malagasy poisonous frogs of genus Mantella are small, diurnal frogs with skin glands containing alkaloids and characterised by aposematic colouration. Due to their noxiousness and warning colouration, it is thought that they do not have many natural predators. Until now, only one successful and one aborted predation on Mantella frogs were reported. Herein, we account about two successful predations on M. aurantiaca in Torotorofotsy wetland, in central-eastern Madagascar. The first predation was observed by lizard Zoonosaurus sp. and the second predation by a snake probably belonging to Thamnosophis lateralis. Both predators did not seem to mind the taste of the M. aurantiaca and ingested it. Keywords. Amphibia: Mantellidae, poison frogs, Thamnosophis, Zoonosaurus Only little is known about predation on poisonous genus Melanophryniscus of southeastern South America, frogs in general, in particular for those containing in Malagasy poison frogs of the genus Mantella (family skin alkaloids. Until now, there are around 30 reports Mantellidae) of Madagascar, and the myobatrachid published on predation on poisonous frogs, mostly genus Pseudophryne of Australia (Daly, Highet and belonging to the families Bufonidae and Leptodactylidae Myers, 1984; Daly et al., 2002). All of -

(Rhacophoridae, Pseudophilautus) in Sri Lanka
Molecular Phylogenetics and Evolution 132 (2019) 14–24 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Diversification of shrub frogs (Rhacophoridae, Pseudophilautus) in Sri Lanka T – Timing and geographic context ⁎ Madhava Meegaskumburaa,b,1, , Gayani Senevirathnec,1, Kelum Manamendra-Arachchid, ⁎ Rohan Pethiyagodae, James Hankenf, Christopher J. Schneiderg, a College of Forestry, Guangxi Key Lab for Forest Ecology and Conservation, Guangxi University, Nanning 530004, PR China b Department of Molecular Biology & Biotechnology, Faculty of Science, University of Peradeniya, Peradeniya, Sri Lanka c Department of Organismal Biology & Anatomy, University of Chicago, Chicago, IL, USA d Postgraduate Institute of Archaeology, Colombo 07, Sri Lanka e Ichthyology Section, Australian Museum, Sydney, NSW 2010, Australia f Museum of Comparative Zoology, Harvard University, Cambridge, MA 02138, USA g Department of Biology, Boston University, Boston, MA 02215, USA ARTICLE INFO ABSTRACT Keywords: Pseudophilautus comprises an endemic diversification predominantly associated with the wet tropical regions ofSri Ancestral-area reconstruction Lanka that provides an opportunity to examine the effects of geography and historical climate change on diversi- Biogeography fication. Using a time-calibrated multi-gene phylogeny, we analyze the tempo of diversification in thecontextof Ecological opportunity past climate and geography to identify historical drivers of current patterns of diversity and distribution. Molecular Diversification dating suggests that the diversification was seeded by migration across a land-bridge connection from India duringa Molecular dating period of climatic cooling and drying, the Oi-1 glacial maximum around the Eocene-Oligocene boundary. Lineage- Speciation through-time plots suggest a gradual and constant rate of diversification, beginning in the Oligocene and extending through the late Miocene and early Pliocene with a slight burst in the Pleistocene. -

Anura, Rhacophoridae)
Zoologica Scripta Patterns of reproductive-mode evolution in Old World tree frogs (Anura, Rhacophoridae) MADHAVA MEEGASKUMBURA,GAYANI SENEVIRATHNE,S.D.BIJU,SONALI GARG,SUYAMA MEEGASKUMBURA,ROHAN PETHIYAGODA,JAMES HANKEN &CHRISTOPHER J. SCHNEIDER Submitted: 3 December 2014 Meegaskumbura, M., Senevirathne, G., Biju, S. D., Garg, S., Meegaskumbura, S., Pethiya- Accepted: 7 May 2015 goda, R., Hanken, J., Schneider, C. J. (2015). Patterns of reproductive-mode evolution in doi:10.1111/zsc.12121 Old World tree frogs (Anura, Rhacophoridae). —Zoologica Scripta, 00, 000–000. The Old World tree frogs (Anura: Rhacophoridae), with 387 species, display a remarkable diversity of reproductive modes – aquatic breeding, terrestrial gel nesting, terrestrial foam nesting and terrestrial direct development. The evolution of these modes has until now remained poorly studied in the context of recent phylogenies for the clade. Here, we use newly obtained DNA sequences from three nuclear and two mitochondrial gene fragments, together with previously published sequence data, to generate a well-resolved phylogeny from which we determine major patterns of reproductive-mode evolution. We show that basal rhacophorids have fully aquatic eggs and larvae. Bayesian ancestral-state reconstruc- tions suggest that terrestrial gel-encapsulated eggs, with early stages of larval development completed within the egg outside of water, are an intermediate stage in the evolution of ter- restrial direct development and foam nesting. The ancestral forms of almost all currently recognized genera (except the fully aquatic basal forms) have a high likelihood of being ter- restrial gel nesters. Direct development and foam nesting each appear to have evolved at least twice within Rhacophoridae, suggesting that reproductive modes are labile and may arise multiple times independently. -

Historically, Direct-Developing Frogs of The
Historically, direct-developing frogs of the genus Eleutherodacylus (Family: Eleutherodactylidae) have been some of the most perplexing and taxonomically challenging amphibians in the New World to investigate. In the following paper, the authors studied these frogs in western Mexico, and conducted a series of morphological, molecular, and vocalization analyses. Their results revealed the existence of six new species. Pictured here is an individual from Ixtlahuacán, Colima, in its natural habitat, one of the species being described. ' © Christoph I. Grünwald 6 Grünwald et al. Six new species of Eleutherodactylus www.mesoamericanherpetology.com www.eaglemountainpublishing.com Version of record: urn:lsid:zoobank.org:pub:3EDC9AB7-94EE-4EAC-89B4-19F5218A593A Six new species of Eleutherodactylus (Anura: Eleutherodactylidae: subgenus Syrrhophus) from Mexico, with a discussion of their systematic relationships and the validity of related species CHRISTOPH I. GRÜNWALD1,2,3,6, JACOBO REYES-VELASCO3,4,6, HECTOR FRANZ-CHÁVEZ3,5,6, KAREN I. 3,6 3,5,6 2,3,6 4 MORALES-FLORES , IVÁN T. AHUMADA-CARRILLO , JASON M. JONES , AND STEPHANE BOISSINOT 1Biencom Real Estate, Carretera Chapala - Jocotepec #57-1, C.P. 45920, Ajijic, Jalisco, Mexico. E-mail: [email protected] (Corresponding author) 2Herpetological Conservation International - Mesoamerica Division, 450 Jolina Way, Encinitas, California 92024, United States. 3Biodiversa A. C., Chapala, Jalisco, Mexico. 4New York University Abu Dhabi, Saadiyat Island, Abu Dhabi, United Arab Emirates. 5Centro Universitario de Ciencias Biológicas y Agropecuarias, Carretera a Nogales Km. 15.5. Las Agujas, Nextipac, Zapopan, C.P. 45110, Jalisco, Mexico. 6Herp.mx A.C., Villa de Alvarez, Colima, Mexico. ABSTRACT: We present an analysis of morphological, molecular, and advertisement call data from sampled populations of Eleutherodactylus (subgenus Syrrhophus) from western Mexico and describe six new spe- cies. -

Biology and Impacts of Pacific Island Invasive Species. 8
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Publications Plant Health Inspection Service 2012 Biology and Impacts of Pacific Island Invasive Species. 8. Eleutherodactylus planirostris, the Greenhouse Frog (Anura: Eleutherodactylidae) Christina A. Olson Utah State University, [email protected] Karen H. Beard Utah State University, [email protected] William C. Pitt National Wildlife Research Center, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/icwdm_usdanwrc Olson, Christina A.; Beard, Karen H.; and Pitt, William C., "Biology and Impacts of Pacific Island Invasive Species. 8. Eleutherodactylus planirostris, the Greenhouse Frog (Anura: Eleutherodactylidae)" (2012). USDA National Wildlife Research Center - Staff Publications. 1174. https://digitalcommons.unl.edu/icwdm_usdanwrc/1174 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Animal and Plant Health Inspection Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA National Wildlife Research Center - Staff Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Biology and Impacts of Pacific Island Invasive Species. 8. Eleutherodactylus planirostris, the Greenhouse Frog (Anura: Eleutherodactylidae)1 Christina A. Olson,2 Karen H. Beard,2,4 and William C. Pitt 3 Abstract: The greenhouse frog, Eleutherodactylus planirostris, is a direct- developing (i.e., no aquatic stage) frog native to Cuba and the Bahamas. It was introduced to Hawai‘i via nursery plants in the early 1990s and then subsequently from Hawai‘i to Guam in 2003. The greenhouse frog is now widespread on five Hawaiian Islands and Guam. -

Amphibia: Anura: Eleutherodactylidae), from Eastern Cuba
124 SOLENODON 12: 124-135, 2015 Another new cryptic frog related to Eleutherodactylus varleyi Dunn (Amphibia: Anura: Eleutherodactylidae), from eastern Cuba Luis M. DÍAZ* and S. Blair HEDGES** *Museo Nacional de Historia Natural de Cuba, Obispo #61, Esquina Oficios, Plaza de Armas, Habana Vieja, CP 10100, Cuba. [email protected] **Department of Biology, 208 Mueller Laboratory, Pennsylvania State University, University Park, Pennsylvania 16802-530, USA. [email protected] ABSTRacT. A new cryptic frog, Eleutherodactylus beguei sp. nov., is described from the pine forests of La Munición, Yateras, Guantánamo Province, Cuba. It is sympatric with E. feichtin- geri, another recently described grass frog closely related to E. varleyi, but differs in morphol- ogy, vocalization and DNA sequences of the mitochondrial Cyt-b gene. One female of the new species was found vocalizing in response to a calling male, a behavior that is still poorly documented in anurans. Same male and female were found in axillary amplexus and sur- rounded by 9 eggs (3.5–3.7 mm in diameter) 5 hours after being isolated in a small container. Key words: Amphibia, Anura, Eleutherodactylidae, Eleutherodactylus, new species, Terrarana, Euhyas, West Indies, Guantánamo, female reciprocation calls, eggs. INtrODUCtION After a recent review of the geographic variation of the Cuban Grass Frog Eleutherodactylus varleyi Dunn, Díaz et al. (2012) described E. feichtingeri, a cryptic species widely distributed in central and eastern Cuba. the two species differ primarily in tympanum size, supratympanic stripe pattern, and advertisement calls. Species recognition was also supported by genetic and cytogenetic data. One of the authors (SBH) conducted DNA sequence analyses that confirmed the existence of two species at La Munición, Humboldt National Park. -
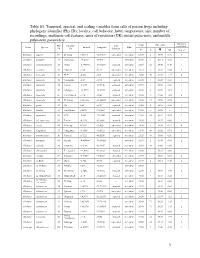
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance
Chapter 21 A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance Juan C. Santos , Rebecca D. Tarvin , and Lauren A. O’Connell 21.1 Introduction Chemical defense has evolved multiple times in nearly every major group of life, from snakes and insects to bacteria and plants (Mebs 2002 ). However, among land vertebrates, chemical defenses are restricted to a few monophyletic groups (i.e., clades). Most of these are amphibians and snakes, but a few rare origins (e.g., Pitohui birds) have stimulated research on acquired chemical defenses (Dumbacher et al. 1992 ). Selective pressures that lead to defense are usually associated with an organ- ism’s limited ability to escape predation or conspicuous behaviors and phenotypes that increase detectability by predators (e.g., diurnality or mating calls) (Speed and Ruxton 2005 ). Defended organisms frequently evolve warning signals to advertise their defense, a phenomenon known as aposematism (Mappes et al. 2005 ). Warning signals such as conspicuous coloration unambiguously inform predators that there will be a substantial cost if they proceed with attack or consumption of the defended prey (Mappes et al. 2005 ). However, aposematism is likely more complex than the simple pairing of signal and defense, encompassing a series of traits (i.e., the apose- matic syndrome) that alter morphology, physiology, and behavior (Mappes and J. C. Santos (*) Department of Zoology, Biodiversity Research Centre , University of British Columbia , #4200-6270 University Blvd , Vancouver , BC , Canada , V6T 1Z4 e-mail: [email protected] R. D. Tarvin University of Texas at Austin , 2415 Speedway Stop C0990 , Austin , TX 78712 , USA e-mail: [email protected] L. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

F3999f15-C572-46Ad-Bbbe
THE STATUTES OF THE REPUBLIC OF SINGAPORE ENDANGERED SPECIES (IMPORT AND EXPORT) ACT (CHAPTER 92A) (Original Enactment: Act 5 of 2006) REVISED EDITION 2008 (1st January 2008) Prepared and Published by THE LAW REVISION COMMISSION UNDER THE AUTHORITY OF THE REVISED EDITION OF THE LAWS ACT (CHAPTER 275) Informal Consolidation – version in force from 22/6/2021 CHAPTER 92A 2008 Ed. Endangered Species (Import and Export) Act ARRANGEMENT OF SECTIONS PART I PRELIMINARY Section 1. Short title 2. Interpretation 3. Appointment of Director-General and authorised officers PART II CONTROL OF IMPORT, EXPORT, ETC., OF SCHEDULED SPECIES 4. Restriction on import, export, etc., of scheduled species 5. Control of scheduled species in transit 6. Defence to offence under section 4 or 5 7. Issue of permit 8. Cancellation of permit PART III ENFORCEMENT POWERS AND PROCEEDINGS 9. Power of inspection 10. Power to investigate and require information 11. Power of entry, search and seizure 12. Powers ancillary to inspections and searches 13. Power to require scheduled species to be marked, etc. 14. Power of arrest 15. Forfeiture 16. Obstruction 17. Penalty for false declarations, etc. 18. General penalty 19. Abetment of offences 20. Offences by bodies corporate, etc. 1 Informal Consolidation – version in force from 22/6/2021 Endangered Species (Import and 2008 Ed. Export) CAP. 92A 2 PART IV MISCELLANEOUS Section 21. Advisory Committee 22. Fees, etc., payable to Board 23. Board not liable for damage caused to goods or property as result of search, etc. 24. Jurisdiction of court, etc. 25. Composition of offences 26. Exemption 27. Service of documents 28. -

Zootaxa, Revision of the Ranitomeya Fantastica Species Complex with Description Of
TERM OF USE This pdf is provided by Magnolia Press for private/research use. Commercial sale or deposition in a public library or website site is prohibited. Zootaxa 1823: 1–24 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) Revision of the Ranitomeya fantastica species complex with description of two new species from Central Peru (Anura: Dendrobatidae) JASON L. BROWN1,4, EVAN TWOMEY1,5, MARK PEPPER2 & MANUEL SANCHEZ RODRIGUEZ3 1Department of Biology, East Carolina University, Greenville, NC, USA 2Understory Enterprises, Charing Cross, Ontario, Canada 3Understory Enterprises, Iquitos, Peru 4Corresponding author. E-mail: [email protected] 5Corresponding author. E-mail:[email protected] Abstract We describe two new species of poison frogs (genus Ranitomeya) from the central Rio Huallaga drainage and adjacent Cordillera Azul in central Peru. Both species were previously considered to be members of Ranitomeya fantastica, a spe- cies described from the town of Yurimaguas, Peru. Extensive sampling of putative R. fantastica (including near-topo- typic material) throughout central Peru, and the resulting morphological and phylogenetic analysis has led us to conclude that R. fantastica sensu lato is a complex of three closely related species rather than a single, widely distributed species. The first of these species occurs near the type locality of R. fantastica but bears significant dissimilarity to the original type series and forms a monophyletic clade that is distributed throughout an expansive lowland zone between Rio Huall- aga and Rio Ucayali. This species is diagnosable by its brilliant red head and advertisement call differences. -

Summary Record of the 26Th Meeting of the Animals Committee
Original language: English AC26 summary record CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-sixth meeting of the Animals Committee Geneva (Switzerland), 15-20 March 2012 and Dublin (Ireland), 22-24 March 2012 SUMMARY RECORD Animals Committee matters 1. Opening of the meeting The Chair opened the meeting and welcomed all participants, before giving the floor to the Secretary- General, who also welcomed everyone and introduced new members of the Secretariat's scientific team (Mr De Meulenaer and Ms Kwitsinskaia) and enforcement team (Ms Garcia Ferreira, Ms Jonsson and Mr van Rensburg). He wished the Committee well in its deliberations. The Chair thanked the Secretary-General and invited suggestions as to how the Conference of the Parties could establish stronger measures to support the Committee as well as export countries, which deserved particular assistance. No other intervention was made during discussion of this item.1 2. Rules of Procedure The Secretariat introduced document AC26 Doc. 2 and proposed amending Rule 22 as follows: “On request, the Secretariat shall distribute printed and translated documents...”. The Secretariat explained that most members regularly indicated that they did not need printed copies and that this proposal was made to reduce costs. Although not opposed to the change in principle, a Party regretted that the suggestion had not been presented in the document, which would have given Parties time to consider it, and was concerned that this unannounced proposal might create a precedent. Another Party asked a question on the procedure to accept observers, but the Chair invited it to raise this topic under agenda item 4 on Admission of observers.