Radiation Synovectomy with 166Ho-Ferric Hydroxide: a First Experience
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effectiveness of Distal Tibial Osteotomy
Nozaka et al. BMC Musculoskeletal Disorders (2020) 21:31 https://doi.org/10.1186/s12891-020-3061-7 RESEARCH ARTICLE Open Access Effectiveness of distal tibial osteotomy with distraction arthroplasty in varus ankle osteoarthritis Koji Nozaka* , Naohisa Miyakoshi, Takeshi Kashiwagura, Yuji Kasukawa, Hidetomo Saito, Hiroaki Kijima, Shuichi Chida, Hiroyuki Tsuchie and Yoichi Shimada Abstract Background: In highly active older individuals, end-stage ankle osteoarthritis has traditionally been treated using tibiotalar arthrodesis, which provides considerable pain relief. However, there is a loss of ankle joint movement and a risk of future arthrosis in the adjacent joints. Distraction arthroplasty is a simple method that allows joint cartilage repair; however, the results are currently mixed, with some reports showing improved pain scores and others showing no improvement. Distal tibial osteotomy (DTO) without fibular osteotomy is a type of joint preservation surgery that has garnered attention in recent years. However, to our knowledge, there are no reports on DTO with joint distraction using a circular external fixator. Therefore, the purpose of this study was to examine the effect of DTO with joint distraction using a circular external fixator for treating ankle osteoarthritis. Methods: A total of 21 patients with medial ankle arthritis were examined. Arthroscopic synovectomy and a microfracture procedure were performed, followed by angled osteotomy and correction of the distal tibia; the ankle joint was then stabilized after its condition improved. An external fixator was used in all patients, and joint distraction of approximately 5.8 mm was performed. All patients were allowed full weight-bearing walking immediately after surgery. Results: The anteroposterior and lateral mortise angle during weight-bearing, talar tilt angle, and anterior translation of the talus on ankle stress radiography were improved significantly (P < 0.05). -

Synovial Fluidfluid 11
LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 253 Aptara Inc CHAPTER SynovialSynovial FluidFluid 11 Key Terms ANTINUCLEAR ANTIBODY ARTHROCENTESIS BULGE TEST CRYSTAL-INDUCED ARTHRITIS GROUND PEPPER HYALURONATE MUCIN OCHRONOTIC SHARDS RHEUMATOID ARTHRITIS (RA) RHEUMATOID FACTOR (RF) RICE BODIES ROPE’S TEST SEPTIC ARTHRITIS Learning Objectives SYNOVIAL SYSTEMIC LUPUS ERYTHEMATOSUS 1. Define synovial. VISCOSITY 2. Describe the formation and function of synovial fluid. 3. Explain the collection and handling of synovial fluid. 4. Describe the appearance of normal and abnormal synovial fluids. 5. Correlate the appearance of synovial fluid with possible cause. 6. Interpret laboratory tests on synovial fluid. 7. Suggest further testing for synovial fluid, based on preliminary results. 8. List the four classes or categories of joint disease. 9. Correlate synovial fluid analyses with their representative disease classification. 253 LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 254 Aptara Inc 254 Graff’s Textbook of Routine Urinalysis and Body Fluids oint fluid is called synovial fluid because of its resem- blance to egg white. It is a viscous, mucinous substance Jthat lubricates most joints. Analysis of synovial fluid is important in the diagnosis of joint disease. Aspiration of joint fluid is indicated for any patient with a joint effusion or inflamed joints. Aspiration of asymptomatic joints is beneficial for patients with gout and pseudogout as these fluids may still contain crystals.1 Evaluation of physical, chemical, and microscopic characteristics of synovial fluid comprise routine analysis. This chapter includes an overview of the composition and function of synovial fluid, and laboratory procedures and their interpretations. -

ICD~10~PCS Complete Code Set Procedural Coding System Sample
ICD~10~PCS Complete Code Set Procedural Coding System Sample Table.of.Contents Preface....................................................................................00 Mouth and Throat ............................................................................. 00 Introducton...........................................................................00 Gastrointestinal System .................................................................. 00 Hepatobiliary System and Pancreas ........................................... 00 What is ICD-10-PCS? ........................................................................ 00 Endocrine System ............................................................................. 00 ICD-10-PCS Code Structure ........................................................... 00 Skin and Breast .................................................................................. 00 ICD-10-PCS Design ........................................................................... 00 Subcutaneous Tissue and Fascia ................................................. 00 ICD-10-PCS Additional Characteristics ...................................... 00 Muscles ................................................................................................. 00 ICD-10-PCS Applications ................................................................ 00 Tendons ................................................................................................ 00 Understandng.Root.Operatons..........................................00 -
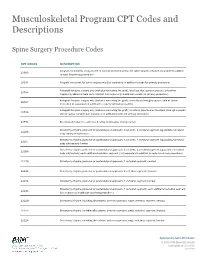
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

CPT® Procedural Coding 110 L with Areportoftheprocedure
20610-20611 2017 Illustrated Coding and Billing Expert for Orthopedics Lower 20610-20611 ICD-9-CM Diagnostic Codes M16.7 Other unilateral secondary 711.05 Pyogenic arthritis involving pelvic osteoarthritis of hip 20610 Arthrocentesis, aspiration and/or region and thigh M17.0 Bilateral primary osteoarthritis of injection, major joint or bursa (eg, 711.06 Pyogenic arthritis involving lower leg knee shoulder, hip, knee, subacromial 713.5 Arthropathy associated with ⇄ M17.11 Unilateral primary osteoarthritis, right bursa); without ultrasound guidance neurological disorders knee 20611 Arthrocentesis, aspiration and/or 714.0 Rheumatoid arthritis ⇄ M17.12 Unilateral primary osteoarthritis, left knee injection, major joint or bursa (eg, 715.15 Osteoarthrosis, localized, primary, pelvic region and thigh M17.2 Bilateral post-traumatic osteoarthritis shoulder, hip, knee, subacromial 715.16 Osteoarthrosis, localized, primary, of knee bursa); with ultrasound guidance, with lower leg M17.5 Other unilateral secondary permanent recording and reporting 715.25 Osteoarthrosis, localized, secondary, osteoarthritis of knee (Do not report 20610, 20611 in pelvic region and thigh ⇄ M1A.051 Idiopathic chronic gout, right hip conjunction with 27370, 76942) 715.26 Osteoarthrosis, localized, secondary, ⇄ M1A.062 Idiopathic chronic gout, left knee (If fluoroscopic, CT, or MRI guidance is lower leg ⇄ M25.052 Hemarthrosis, left hip ⇄ M25.061 Hemarthrosis, right knee performed, see 77002, 77012, 77021) 715.35 Osteoarthrosis, localized, not specified whether primary -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Knee Joint Surgery: Open Synovectomy
Musculoskeletal Surgical Services: Open Surgical Procedures; Knee Joint Surgery: Open Synovectomy POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5588 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from HealthHelp. -

DISSERTATION INVESTIGATION of CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY for the EVALUATION of EQUINE ARTICULAR CARTILAGE Su
DISSERTATION INVESTIGATION OF CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY FOR THE EVALUATION OF EQUINE ARTICULAR CARTILAGE Submitted by Bradley B. Nelson Department of Clinical Sciences In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Fall 2017 Doctoral Committee: Advisor: Christopher E. Kawcak Co-Advisor: Laurie R. Goodrich C. Wayne McIlwraith Mark W. Grinstaff Myra F. Barrett Copyright by Bradley Bernard Nelson 2017 All Rights Reserved ABSTRACT INVESTIGATION OF CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY FOR THE EVALUATION OF EQUINE ARTICULAR CARTILAGE Osteoarthritis and articular cartilage injury are substantial problems in horses causing joint pain, lameness and decreased athleticism resonant of the afflictions that occur in humans. This debilitating joint disease causes progressive articular cartilage degeneration and coupled with a poor capacity to heal necessitates that articular cartilage injury is detected early before irreparable damage ensues. The use of diagnostic imaging is critical to identify and characterize articular cartilage injury, though currently available methods are unable to identify these early degenerative changes. Cationic contrast-enhanced computed tomography (CECT) uses a cationic contrast media (CA4+) to detect the early molecular changes that occur in the extracellular matrix. Glycosaminoglycans (GAGs) within the extracellular matrix are important for the providing the compressive stiffness of articular cartilage and their degradation is an early event in the development of osteoarthritis. Cationic CECT imaging capitalizes on the electrostatic attraction between CA4+ and GAGs; exposing the proportional relationship between the amount of GAGs present within and the amount of CA4+ that diffuses into the tissue. The amount of CA4+ that resides in the tissue is then quantified through CECT imaging and estimates tissue integrity through nondestructive assessment. -

ARTICULAR BLEEDING (HEMARTHROSIS) in HEMOPHILIA an ORTHOPEDIST’S POINT of VIEW Second Edition
TREATMENT OF HEMOPHILIA April 2008 · No. 23 ARTICULAR BLEEDING (HEMARTHROSIS) IN HEMOPHILIA AN ORTHOPEDIST’S POINT OF VIEW Second edition E. C. Rodríguez-Merchán Consultant Orthopedic Surgeon La Paz University Hospital Madrid, Spain Associate Professor of Orthopedics University Autonoma Madrid, Spain Published by the World Federation of Hemophilia (WFH), 2000; revised 2008. © Copyright World Federation of Hemophilia, 2008 The WFH encourages redistribution of its publications for educational purposes by not-for-profit hemophilia organizations. In order to obtain permission to reprint, redistribute, or translate this publication, please contact the Programs and Education Department at the address below. This publication is accessible from the World Federation of Hemophilia’s eLearning Platform at eLearning.wfh.org Additional copies are also available from the WFH at: World Federation of Hemophilia 1425 René Lévesque Boulevard West, Suite 1010 Montréal, Québec H3G 1T7 CANADA Tel. : (514) 875-7944 Fax : (514) 875-8916 E-mail: [email protected] Internet: www.wfh.org The Treatment of Hemophilia series is intended to provide general information on the treatment and management of hemophilia. The World Federation of Hemophilia does not engage in the practice of medicine and under no circumstances recommends particular treatment for specific individuals. Dose schedules and other treatment regimes are continually revised and new side-effects recognized. WFH makes no representation, express or implied, that drug doses or other treatment recommendations in this publication are correct. For these reasons it is strongly recommended that individuals seek the advice of a medical adviser and/or to consult printed instructions provided by the pharmaceutical company before administering any of the drugs referred to in this monograph. -

Joint & Tendon Injection
Coding Corner JOINT & TENDON INJECTION Joint Aspiration/Injection Report only a single unit of a joint injection code (seen on table below) for each joint treated, regardless of how many aspirations and/or injections occur in a single joint. For example, if the physician administers two injections, one on either side of the right knee, you would report 20610 x 1. The Centers for Medicare & Medicaid Services (CMS) instructs that you should also “Indicate which knee was injected by using the RT (right) or LT (left) modifier on the injection procedure.” Code Description 20600 Arthrocentesis, aspiration and/or injection, small joint or bursa (e.g., fingers, toes); without ultrasound guidance 20604 Arthrocentesis, aspiration and/or injection, small joint or bursa (e.g., fingers, toes); with ultrasound guidance, with permanent recording and reporting 20605 Arthrocentesis, aspiration and/or injection, intermediate joint or bursa (e.g., temporomandibular, acromioclavicular, wrist, elbow or ankle, olecranon bursa); without ultrasound guidance 20606 Arthrocentesis, aspiration and/or injection, intermediate joint or bursa (e.g., temporomandibular, acromioclavicular, wrist, elbow or ankle, olecranon bursa); with ultrasound guidance, with permanent recording and reporting 20610 Arthrocentesis, aspiration and/or injection, major joint or bursa (e.g., shoulder, hip, knee, subacromial bursa); without ultrasound guidance 20611 Arthrocentesis, aspiration and/or injection, major joint or bursa (e.g., shoulder, hip, knee, subacromial bursa); with ultrasound guidance, with permanent recording and reporting You may report multiple units only if aspiration/injection is performed in more than one joint (e.g., both knees or left knee and left shoulder). If aspirations and/or injections occur on opposite, paired joints (e.g., both knees), you may report one unit with modifier 50 Bilateral procedure appended, per CMS instruction. -

APG Regulations
FINAL as of 8/22/08 Pursuant to the authority vested in the Commissioner of Health by Section 2807(2-a) of the Public Health Law, Part 86 of Title 10 of the Official Compilation of Codes, Rules and Regulations of the State of New York, is amended by adding a new Subpart 86-8, to be effective upon filing with the Secretary of State, to read as follows: SUBPART 86-8 OUTPATIENT SERVICES: AMBULATORY PATIENT GROUP (Statutory authority: Public Health Law § 2807(2-a)(e)) Sec. 86-8.1 Scope 86-8.2 Definitions 86-8.3 Record keeping, reports and audits 86-8.4 Capital reimbursement 86-8.5 Administrative rate appeals 86-8.6 Rates for new facilities during the transition period 86-8.7 APGs and relative weights 86-8.8 Base rates 86-8.9 Diagnostic coding and rate computation 86-8.10 Exclusions from payment 86-8.11 System updating 86-8.12 Payments for extended hours of operation § 86-8.1 Scope (a) This Subpart shall govern Medicaid rates of payments for ambulatory care services provided in the following categories of facilities for the following periods: (1) outpatient services provided by general hospitals on and after December 1, 2008; (2) emergency department services provided by general hospitals on and after January 1, 2009; (3) ambulatory surgery services provided by general hospitals on and after December 1, 2008; (4) ambulatory services provided by diagnostic and treatment centers on and after March 1, 2009; and (5) ambulatory surgery services provided by free-standing ambulatory surgery centers on and after March 1, 2009. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular