2019 & 2020 Facility Scoring Methodology for Cost & Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effectiveness of Distal Tibial Osteotomy
Nozaka et al. BMC Musculoskeletal Disorders (2020) 21:31 https://doi.org/10.1186/s12891-020-3061-7 RESEARCH ARTICLE Open Access Effectiveness of distal tibial osteotomy with distraction arthroplasty in varus ankle osteoarthritis Koji Nozaka* , Naohisa Miyakoshi, Takeshi Kashiwagura, Yuji Kasukawa, Hidetomo Saito, Hiroaki Kijima, Shuichi Chida, Hiroyuki Tsuchie and Yoichi Shimada Abstract Background: In highly active older individuals, end-stage ankle osteoarthritis has traditionally been treated using tibiotalar arthrodesis, which provides considerable pain relief. However, there is a loss of ankle joint movement and a risk of future arthrosis in the adjacent joints. Distraction arthroplasty is a simple method that allows joint cartilage repair; however, the results are currently mixed, with some reports showing improved pain scores and others showing no improvement. Distal tibial osteotomy (DTO) without fibular osteotomy is a type of joint preservation surgery that has garnered attention in recent years. However, to our knowledge, there are no reports on DTO with joint distraction using a circular external fixator. Therefore, the purpose of this study was to examine the effect of DTO with joint distraction using a circular external fixator for treating ankle osteoarthritis. Methods: A total of 21 patients with medial ankle arthritis were examined. Arthroscopic synovectomy and a microfracture procedure were performed, followed by angled osteotomy and correction of the distal tibia; the ankle joint was then stabilized after its condition improved. An external fixator was used in all patients, and joint distraction of approximately 5.8 mm was performed. All patients were allowed full weight-bearing walking immediately after surgery. Results: The anteroposterior and lateral mortise angle during weight-bearing, talar tilt angle, and anterior translation of the talus on ankle stress radiography were improved significantly (P < 0.05). -

FGM in Canada
Compiled by Patricia Huston MD, MPH Scientific Communications International, Inc for the Federal Interdepartmental Working Group on FGM. Copies of this report are available from: Women's Health Bureau Health Canada [email protected] The Canadian Women's Health Network 203-419 Graham Avenue Winnipeg, Manitoba R3C 0M3 fax: (204)989-2355 The opinions expressed in this report are not necessarily those of the Government of Canada or any of the other organizations represented. Dedication This report is dedicated to all the women in the world who have undergone FGM and to all the people who are helping them live with and reverse this procedure. This report is part of the ongoing commitment of Canadians and the Government of Canada to stop this practice in Canada and to improve the health and well-being of affected women and their communities. Executive Summary Female genital mutilation (FGM), or the ritual excision of part or all of the external female genitalia, is an ancient cultural practice that occurs around the world today, especially in Africa. With recent immigration to Canada of peoples from Somalia, Ethiopia and Eritrea, Sudan and Nigeria, women who have undergone this practice are now increasingly living in Canada. It is firmly believed by the people who practise it, that FGM improves feminine hygiene, that it will help eliminate disease and it is thought to be the only way to preserve family honour, a girl's virginity and her marriageability. FGM has a number of important adverse health effects including risks of infection and excessive bleeding (often performed when a girl is pre-pubertal). -
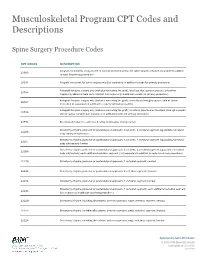
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Knee Joint Surgery: Open Synovectomy
Musculoskeletal Surgical Services: Open Surgical Procedures; Knee Joint Surgery: Open Synovectomy POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5588 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from HealthHelp. -

Core Curriculum for Surgical Technology Sixth Edition
Core Curriculum for Surgical Technology Sixth Edition Core Curriculum 6.indd 1 11/17/10 11:51 PM TABLE OF CONTENTS I. Healthcare sciences A. Anatomy and physiology 7 B. Pharmacology and anesthesia 37 C. Medical terminology 49 D. Microbiology 63 E. Pathophysiology 71 II. Technological sciences A. Electricity 85 B. Information technology 86 C. Robotics 88 III. Patient care concepts A. Biopsychosocial needs of the patient 91 B. Death and dying 92 IV. Surgical technology A. Preoperative 1. Non-sterile a. Attire 97 b. Preoperative physical preparation of the patient 98 c. tneitaP noitacifitnedi 99 d. Transportation 100 e. Review of the chart 101 f. Surgical consent 102 g. refsnarT 104 h. Positioning 105 i. Urinary catheterization 106 j. Skin preparation 108 k. Equipment 110 l. Instrumentation 112 2. Sterile a. Asepsis and sterile technique 113 b. Hand hygiene and surgical scrub 115 c. Gowning and gloving 116 d. Surgical counts 117 e. Draping 118 B. Intraoperative: Sterile 1. Specimen care 119 2. Abdominal incisions 121 3. Hemostasis 122 4. Exposure 123 5. Catheters and drains 124 6. Wound closure 128 7. Surgical dressings 137 8. Wound healing 140 1 c. Light regulation d. Photoreceptors e. Macula lutea f. Fovea centralis g. Optic disc h. Brain pathways C. Ear 1. Anatomy a. External ear (1) Auricle (pinna) (2) Tragus b. Middle ear (1) Ossicles (a) Malleus (b) Incus (c) Stapes (2) Oval window (3) Round window (4) Mastoid sinus (5) Eustachian tube c. Internal ear (1) Labyrinth (2) Cochlea 2. Physiology of hearing a. Sound wave reception b. Bone conduction c. -
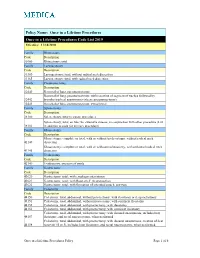
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Radiation Synovectomy with 166Ho-Ferric Hydroxide: a First Experience
Radiation Synovectomy with 166Ho-Ferric Hydroxide: A First Experience Sedat Ofluoglu, MD1; Eva Schwameis, MD2; Harald Zehetgruber, MD2; Ernst Havlik, PhD3; Axel Wanivenhaus, MD2; Ingrid Schweeger, MD1; Konrad Weiss, MD4; Helmut Sinzinger, MD1; and Christian Pirich, MD1 1Department of Nuclear Medicine, University of Vienna, Vienna, Austria; 2Department of Orthopedics, University of Vienna, Vienna, Austria; 3Department of Biomedical Engineering and Physics and Ludwig Boltzmann Institute of Nuclear Medicine, Vienna, Austria; and 4Department of Nuclear Medicine, General Hospital of Wiener Neustadt, Wiener Neustadt, Austria lage, leading to the progressive loss of joint function and Radiation synovectomy (RS) is indicated when conventional significant disability. Treatment of chronic synovitis using pharmacologic treatment of chronic synovitis has not relieved radiation synovectomy (RS) aims to stop the inflammatory its symptoms. The use of radionuclides that are bound to ferric process causing pain, disability, and nonreversible structural hydroxide (FH) particles has been shown to be effective and damage to the joint (1–3). RS has been in clinical use for 166 safe for this procedure. Ho-FH macroaggregates offer prom- 50y(4) primarily as an alternative to surgical treatment (5). ising properties for RS but there is a lack of clinical data. We Safety is one of the most important aspects when radionu- investigated the efficacy and safety of 166Ho-FH in a prospective clinical trial in patients suffering from chronic synovitis. Meth- clides are applied therapeutically. The use of ferric hydrox- ods: Twenty-four intraarticular injections were performed in 22 ide (FH) particles as a carrier may offer some advantages patients receiving a mean activity of 1.11 GBq (range, 0.77–1.24 over other carriers with respect to the frequency and degree GBq) 166Ho-FH. -

GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017
UnitedHealthcare® Commercial Medical Policy GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017 Table of Contents Page Related Commercial Policy INSTRUCTIONS FOR USE .......................................... 1 Blepharoplasty, Blepharoptosis and Brow Ptosis BENEFIT CONSIDERATIONS ...................................... 1 Repair COVERAGE RATIONALE ............................................. 2 Botulinum Toxin A and B DEFINITIONS .......................................................... 3 Cosmetic and Reconstructive Procedures APPLICABLE CODES ................................................. 4 DESCRIPTION OF SERVICES ...................................... 8 Gonadotropin Releasing Hormone Analogs CLINICAL EVIDENCE ................................................. 8 Panniculectomy and Body Contouring Procedures U.S. FOOD AND DRUG ADMINISTRATION ................... 11 Rhinoplasty and Other Nasal Surgeries CENTERS FOR MEDICARE AND MEDICAID SERVICES ... 11 Speech Language Pathology Services REFERENCES .......................................................... 11 POLICY HISTORY/REVISION INFORMATION ................ 12 Community Plan Policy Gender Dysphoria Treatment Related Optum Guideline Gender Dysphoria INSTRUCTIONS FOR USE This Medical Policy provides assistance in interpreting UnitedHealthcare benefit plans. When deciding coverage, the member specific benefit plan document must be referenced. The terms of the member specific benefit plan document [e.g., Certificate of Coverage (COC), Schedule of -

April 2013, Volume 5, Issue 4
VOLUME 5 MONTHLY ISSUE MEDICAL STAFF 4 NEWSLETTER ProgressNotes April TORRANCE MEMORIAL MEDICAL CENTER 2013 this issue CPOE P.1 Doctor’s Day P.2 MEC Approvals P.3-4 Medical Staff Calendar P.5 New Providers P.6 Roster Updates P.6 What is it? CPOE Select Rewards is a tiered program geared to reward pro- CPOE viders as they expand their use of CPOE (Computerized Provider Order Entry). Computerized How are providers enrolled in the CPOE Select Rewards Provider Order Program? Entry Select Enrollment is automatic as you or your group begins CPOE. Rewards Program How does it work? Providers qualify for monthly raffles based on meeting or exceed- ing CPOE percentage goals for their assigned tier. When will winners be announced? Winners will be announced the second Monday of every month, beginning in April. CPOE Monthly Raffle includes several prizes including an i-Pad mini. Individualized program details will be sent out to providers. Please call CPOE hotline at x2763 (xCPOE) if you have any questions. Doctor’s Day Thank you to all the physicians who attended the Doctor’s Day Celebration at Torrance Memorial on Wednesday, March 27th. The event was a success with many physicians attending to enjoy a great meal, music and prizes. Congratulations to Dr. Lauren Nguyen and Dr. James Flores, the two lucky winners of the iPad mini and the thirty physicians who also won gift cards to Nordstroms, California Pizza Kitchen, HealthLinks, Starbucks and iTunes. Torrance Memorial appreciates our physicians’ dedication and commitment to the health of our community. Medical Executive Committee Approvals The following items were presented and actions were approved at the March 12, 2013 Medical Executive Committee meeting: A. -

Gender Dysphoria Treatment – Commercial Medical Policy
UnitedHealthcare® Commercial Medical Policy Gender Dysphoria Treatment Policy Number: 2021T0580J Effective Date: April 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policies Coverage Rationale ....................................................................... 1 • Blepharoplasty, Blepharoptosis and Brow Ptosis Documentation Requirements ...................................................... 3 Repair Definitions ...................................................................................... 4 • Botulinum Toxins A and B Applicable Codes .......................................................................... 5 • Cosmetic and Reconstructive Procedures Description of Services ................................................................. 9 • Gonadotropin Releasing Hormone Analogs Benefit Considerations .................................................................. 9 Clinical Evidence ......................................................................... 10 • Habilitative Services and Outpatient Rehabilitation U.S. Food and Drug Administration ........................................... 15 Therapy References ................................................................................... 15 • Panniculectomy and Body Contouring Procedures Policy History/Revision Information ........................................... 16 • Rhinoplasty and Other Nasal Surgeries Instructions for Use ..................................................................... 17 Community Plan Policy • Gender Dysphoria -

ARTICULAR BLEEDING (HEMARTHROSIS) in HEMOPHILIA an ORTHOPEDIST’S POINT of VIEW Second Edition
TREATMENT OF HEMOPHILIA April 2008 · No. 23 ARTICULAR BLEEDING (HEMARTHROSIS) IN HEMOPHILIA AN ORTHOPEDIST’S POINT OF VIEW Second edition E. C. Rodríguez-Merchán Consultant Orthopedic Surgeon La Paz University Hospital Madrid, Spain Associate Professor of Orthopedics University Autonoma Madrid, Spain Published by the World Federation of Hemophilia (WFH), 2000; revised 2008. © Copyright World Federation of Hemophilia, 2008 The WFH encourages redistribution of its publications for educational purposes by not-for-profit hemophilia organizations. In order to obtain permission to reprint, redistribute, or translate this publication, please contact the Programs and Education Department at the address below. This publication is accessible from the World Federation of Hemophilia’s eLearning Platform at eLearning.wfh.org Additional copies are also available from the WFH at: World Federation of Hemophilia 1425 René Lévesque Boulevard West, Suite 1010 Montréal, Québec H3G 1T7 CANADA Tel. : (514) 875-7944 Fax : (514) 875-8916 E-mail: [email protected] Internet: www.wfh.org The Treatment of Hemophilia series is intended to provide general information on the treatment and management of hemophilia. The World Federation of Hemophilia does not engage in the practice of medicine and under no circumstances recommends particular treatment for specific individuals. Dose schedules and other treatment regimes are continually revised and new side-effects recognized. WFH makes no representation, express or implied, that drug doses or other treatment recommendations in this publication are correct. For these reasons it is strongly recommended that individuals seek the advice of a medical adviser and/or to consult printed instructions provided by the pharmaceutical company before administering any of the drugs referred to in this monograph.