ARTICULAR BLEEDING (HEMARTHROSIS) in HEMOPHILIA an ORTHOPEDIST’S POINT of VIEW Second Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WFH Treatment Guidelines 3Ed Chapter 7 Treatment Of
96 TREATMENT OF SPECIFIC 7 HEMORRHAGES Johnny Mahlangu1 | Gerard Dolan2 | Alison Dougall3 | Nicholas J. Goddard4 | Enrique D. Preza Hernández5 | Margaret V. Ragni6 | Bradley Rayner7 | Jerzy Windyga8 | Glenn F. Pierce9 | Alok Srivastava10 1 Department of Molecular Medicine and Haematology, University of the Witwatersrand, National Health Laboratory Service, Johannesburg, South Africa 2 Guy’s and St. Thomas’ Hospitals NHS Foundation Trust, London, UK 3 Special Care Dentistry Division of Child and Public Dental Health, School of Dental Science, Trinity College Dublin, Dublin Dental University Hospital, Dublin, Ireland 4 Department of Trauma and Orthopaedics, Royal Free Hospital, London, UK 5 Mexico City, Mexico 6 Division of Hematology/Oncology, Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA 7 Cape Town, South Africa 8 Department of Hemostasis Disorders and Internal Medicine, Laboratory of Hemostasis and Metabolic Diseases, Institute of Hematology and Transfusion Medicine, Warsaw, Poland 9 World Federation of Hemophilia, Montreal, QC, Canada 10 Department of Haematology, Christian Medical College, Vellore, India All statements identified as recommendations are • In general, the main treatment for bleeding episodes in consensus based, as denoted by CB. patients with severe hemophilia is prompt clotting factor replacement therapy and rehabilitation. However, different types of bleeds and bleeding at particular anatomical sites 7.1 Introduction may require more specific management with additional -

Effectiveness of Distal Tibial Osteotomy
Nozaka et al. BMC Musculoskeletal Disorders (2020) 21:31 https://doi.org/10.1186/s12891-020-3061-7 RESEARCH ARTICLE Open Access Effectiveness of distal tibial osteotomy with distraction arthroplasty in varus ankle osteoarthritis Koji Nozaka* , Naohisa Miyakoshi, Takeshi Kashiwagura, Yuji Kasukawa, Hidetomo Saito, Hiroaki Kijima, Shuichi Chida, Hiroyuki Tsuchie and Yoichi Shimada Abstract Background: In highly active older individuals, end-stage ankle osteoarthritis has traditionally been treated using tibiotalar arthrodesis, which provides considerable pain relief. However, there is a loss of ankle joint movement and a risk of future arthrosis in the adjacent joints. Distraction arthroplasty is a simple method that allows joint cartilage repair; however, the results are currently mixed, with some reports showing improved pain scores and others showing no improvement. Distal tibial osteotomy (DTO) without fibular osteotomy is a type of joint preservation surgery that has garnered attention in recent years. However, to our knowledge, there are no reports on DTO with joint distraction using a circular external fixator. Therefore, the purpose of this study was to examine the effect of DTO with joint distraction using a circular external fixator for treating ankle osteoarthritis. Methods: A total of 21 patients with medial ankle arthritis were examined. Arthroscopic synovectomy and a microfracture procedure were performed, followed by angled osteotomy and correction of the distal tibia; the ankle joint was then stabilized after its condition improved. An external fixator was used in all patients, and joint distraction of approximately 5.8 mm was performed. All patients were allowed full weight-bearing walking immediately after surgery. Results: The anteroposterior and lateral mortise angle during weight-bearing, talar tilt angle, and anterior translation of the talus on ankle stress radiography were improved significantly (P < 0.05). -

Familial Multiple Coagulation Factor Deficiencies
Journal of Clinical Medicine Article Familial Multiple Coagulation Factor Deficiencies (FMCFDs) in a Large Cohort of Patients—A Single-Center Experience in Genetic Diagnosis Barbara Preisler 1,†, Behnaz Pezeshkpoor 1,† , Atanas Banchev 2 , Ronald Fischer 3, Barbara Zieger 4, Ute Scholz 5, Heiko Rühl 1, Bettina Kemkes-Matthes 6, Ursula Schmitt 7, Antje Redlich 8 , Sule Unal 9 , Hans-Jürgen Laws 10, Martin Olivieri 11 , Johannes Oldenburg 1 and Anna Pavlova 1,* 1 Institute of Experimental Hematology and Transfusion Medicine, University Clinic Bonn, 53127 Bonn, Germany; [email protected] (B.P.); [email protected] (B.P.); [email protected] (H.R.); [email protected] (J.O.) 2 Department of Paediatric Haematology and Oncology, University Hospital “Tzaritza Giovanna—ISUL”, 1527 Sofia, Bulgaria; [email protected] 3 Hemophilia Care Center, SRH Kurpfalzkrankenhaus Heidelberg, 69123 Heidelberg, Germany; ronald.fi[email protected] 4 Department of Pediatrics and Adolescent Medicine, University Medical Center–University of Freiburg, 79106 Freiburg, Germany; [email protected] 5 Center of Hemostasis, MVZ Labor Leipzig, 04289 Leipzig, Germany; [email protected] 6 Hemostasis Center, Justus Liebig University Giessen, 35392 Giessen, Germany; [email protected] 7 Center of Hemostasis Berlin, 10789 Berlin-Schöneberg, Germany; [email protected] 8 Pediatric Oncology Department, Otto von Guericke University Children’s Hospital Magdeburg, 39120 Magdeburg, Germany; [email protected] 9 Division of Pediatric Hematology Ankara, Hacettepe University, 06100 Ankara, Turkey; Citation: Preisler, B.; Pezeshkpoor, [email protected] B.; Banchev, A.; Fischer, R.; Zieger, B.; 10 Department of Pediatric Oncology, Hematology and Clinical Immunology, University of Duesseldorf, Scholz, U.; Rühl, H.; Kemkes-Matthes, 40225 Duesseldorf, Germany; [email protected] B.; Schmitt, U.; Redlich, A.; et al. -

Synovial Fluidfluid 11
LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 253 Aptara Inc CHAPTER SynovialSynovial FluidFluid 11 Key Terms ANTINUCLEAR ANTIBODY ARTHROCENTESIS BULGE TEST CRYSTAL-INDUCED ARTHRITIS GROUND PEPPER HYALURONATE MUCIN OCHRONOTIC SHARDS RHEUMATOID ARTHRITIS (RA) RHEUMATOID FACTOR (RF) RICE BODIES ROPE’S TEST SEPTIC ARTHRITIS Learning Objectives SYNOVIAL SYSTEMIC LUPUS ERYTHEMATOSUS 1. Define synovial. VISCOSITY 2. Describe the formation and function of synovial fluid. 3. Explain the collection and handling of synovial fluid. 4. Describe the appearance of normal and abnormal synovial fluids. 5. Correlate the appearance of synovial fluid with possible cause. 6. Interpret laboratory tests on synovial fluid. 7. Suggest further testing for synovial fluid, based on preliminary results. 8. List the four classes or categories of joint disease. 9. Correlate synovial fluid analyses with their representative disease classification. 253 LWBK461-c11_p253-262.qxd 11/18/09 6:04 PM Page 254 Aptara Inc 254 Graff’s Textbook of Routine Urinalysis and Body Fluids oint fluid is called synovial fluid because of its resem- blance to egg white. It is a viscous, mucinous substance Jthat lubricates most joints. Analysis of synovial fluid is important in the diagnosis of joint disease. Aspiration of joint fluid is indicated for any patient with a joint effusion or inflamed joints. Aspiration of asymptomatic joints is beneficial for patients with gout and pseudogout as these fluids may still contain crystals.1 Evaluation of physical, chemical, and microscopic characteristics of synovial fluid comprise routine analysis. This chapter includes an overview of the composition and function of synovial fluid, and laboratory procedures and their interpretations. -

ICD~10~PCS Complete Code Set Procedural Coding System Sample
ICD~10~PCS Complete Code Set Procedural Coding System Sample Table.of.Contents Preface....................................................................................00 Mouth and Throat ............................................................................. 00 Introducton...........................................................................00 Gastrointestinal System .................................................................. 00 Hepatobiliary System and Pancreas ........................................... 00 What is ICD-10-PCS? ........................................................................ 00 Endocrine System ............................................................................. 00 ICD-10-PCS Code Structure ........................................................... 00 Skin and Breast .................................................................................. 00 ICD-10-PCS Design ........................................................................... 00 Subcutaneous Tissue and Fascia ................................................. 00 ICD-10-PCS Additional Characteristics ...................................... 00 Muscles ................................................................................................. 00 ICD-10-PCS Applications ................................................................ 00 Tendons ................................................................................................ 00 Understandng.Root.Operatons..........................................00 -
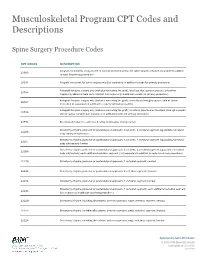
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Immune-Pathophysiology and -Therapy of Childhood Purpura
Egypt J Pediatr Allergy Immunol 2009;7(1):3-13. Review article Immune-pathophysiology and -therapy of childhood purpura Safinaz A Elhabashy Professor of Pediatrics, Ain Shams University, Cairo Childhood purpura - Overview vasculitic disorders present with palpable Purpura (from the Latin, purpura, meaning purpura2. Purpura may be secondary to "purple") is the appearance of red or purple thrombocytopenia, platelet dysfunction, discolorations on the skin that do not blanch on coagulation factor deficiency or vascular defect as applying pressure. They are caused by bleeding shown in table 1. underneath the skin. Purpura measure 0.3-1cm, A thorough history (Table 2) and a careful while petechiae measure less than 3mm and physical examination (Table 3) are critical first ecchymoses greater than 1cm1. The integrity of steps in the evaluation of children with purpura3. the vascular system depends on three interacting When the history and physical examination elements: platelets, plasma coagulation factors suggest the presence of a bleeding disorder, and blood vessels. All three elements are required laboratory screening studies may include a for proper hemostasis, but the pattern of bleeding complete blood count, peripheral blood smear, depends to some extent on the specific defect. In prothrombin time (PT) and activated partial general, platelet disorders manifest petechiae, thromboplastin time (aPTT). With few exceptions, mucosal bleeding (wet purpura) or, rarely, central these studies should identify most hemostatic nervous system bleeding; -

CPT® Procedural Coding 110 L with Areportoftheprocedure
20610-20611 2017 Illustrated Coding and Billing Expert for Orthopedics Lower 20610-20611 ICD-9-CM Diagnostic Codes M16.7 Other unilateral secondary 711.05 Pyogenic arthritis involving pelvic osteoarthritis of hip 20610 Arthrocentesis, aspiration and/or region and thigh M17.0 Bilateral primary osteoarthritis of injection, major joint or bursa (eg, 711.06 Pyogenic arthritis involving lower leg knee shoulder, hip, knee, subacromial 713.5 Arthropathy associated with ⇄ M17.11 Unilateral primary osteoarthritis, right bursa); without ultrasound guidance neurological disorders knee 20611 Arthrocentesis, aspiration and/or 714.0 Rheumatoid arthritis ⇄ M17.12 Unilateral primary osteoarthritis, left knee injection, major joint or bursa (eg, 715.15 Osteoarthrosis, localized, primary, pelvic region and thigh M17.2 Bilateral post-traumatic osteoarthritis shoulder, hip, knee, subacromial 715.16 Osteoarthrosis, localized, primary, of knee bursa); with ultrasound guidance, with lower leg M17.5 Other unilateral secondary permanent recording and reporting 715.25 Osteoarthrosis, localized, secondary, osteoarthritis of knee (Do not report 20610, 20611 in pelvic region and thigh ⇄ M1A.051 Idiopathic chronic gout, right hip conjunction with 27370, 76942) 715.26 Osteoarthrosis, localized, secondary, ⇄ M1A.062 Idiopathic chronic gout, left knee (If fluoroscopic, CT, or MRI guidance is lower leg ⇄ M25.052 Hemarthrosis, left hip ⇄ M25.061 Hemarthrosis, right knee performed, see 77002, 77012, 77021) 715.35 Osteoarthrosis, localized, not specified whether primary -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Knee Joint Surgery: Open Synovectomy
Musculoskeletal Surgical Services: Open Surgical Procedures; Knee Joint Surgery: Open Synovectomy POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5588 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from HealthHelp. -

Radiation Synovectomy with 166Ho-Ferric Hydroxide: a First Experience
Radiation Synovectomy with 166Ho-Ferric Hydroxide: A First Experience Sedat Ofluoglu, MD1; Eva Schwameis, MD2; Harald Zehetgruber, MD2; Ernst Havlik, PhD3; Axel Wanivenhaus, MD2; Ingrid Schweeger, MD1; Konrad Weiss, MD4; Helmut Sinzinger, MD1; and Christian Pirich, MD1 1Department of Nuclear Medicine, University of Vienna, Vienna, Austria; 2Department of Orthopedics, University of Vienna, Vienna, Austria; 3Department of Biomedical Engineering and Physics and Ludwig Boltzmann Institute of Nuclear Medicine, Vienna, Austria; and 4Department of Nuclear Medicine, General Hospital of Wiener Neustadt, Wiener Neustadt, Austria lage, leading to the progressive loss of joint function and Radiation synovectomy (RS) is indicated when conventional significant disability. Treatment of chronic synovitis using pharmacologic treatment of chronic synovitis has not relieved radiation synovectomy (RS) aims to stop the inflammatory its symptoms. The use of radionuclides that are bound to ferric process causing pain, disability, and nonreversible structural hydroxide (FH) particles has been shown to be effective and damage to the joint (1–3). RS has been in clinical use for 166 safe for this procedure. Ho-FH macroaggregates offer prom- 50y(4) primarily as an alternative to surgical treatment (5). ising properties for RS but there is a lack of clinical data. We Safety is one of the most important aspects when radionu- investigated the efficacy and safety of 166Ho-FH in a prospective clinical trial in patients suffering from chronic synovitis. Meth- clides are applied therapeutically. The use of ferric hydrox- ods: Twenty-four intraarticular injections were performed in 22 ide (FH) particles as a carrier may offer some advantages patients receiving a mean activity of 1.11 GBq (range, 0.77–1.24 over other carriers with respect to the frequency and degree GBq) 166Ho-FH. -

DISSERTATION INVESTIGATION of CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY for the EVALUATION of EQUINE ARTICULAR CARTILAGE Su
DISSERTATION INVESTIGATION OF CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY FOR THE EVALUATION OF EQUINE ARTICULAR CARTILAGE Submitted by Bradley B. Nelson Department of Clinical Sciences In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Fall 2017 Doctoral Committee: Advisor: Christopher E. Kawcak Co-Advisor: Laurie R. Goodrich C. Wayne McIlwraith Mark W. Grinstaff Myra F. Barrett Copyright by Bradley Bernard Nelson 2017 All Rights Reserved ABSTRACT INVESTIGATION OF CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY FOR THE EVALUATION OF EQUINE ARTICULAR CARTILAGE Osteoarthritis and articular cartilage injury are substantial problems in horses causing joint pain, lameness and decreased athleticism resonant of the afflictions that occur in humans. This debilitating joint disease causes progressive articular cartilage degeneration and coupled with a poor capacity to heal necessitates that articular cartilage injury is detected early before irreparable damage ensues. The use of diagnostic imaging is critical to identify and characterize articular cartilage injury, though currently available methods are unable to identify these early degenerative changes. Cationic contrast-enhanced computed tomography (CECT) uses a cationic contrast media (CA4+) to detect the early molecular changes that occur in the extracellular matrix. Glycosaminoglycans (GAGs) within the extracellular matrix are important for the providing the compressive stiffness of articular cartilage and their degradation is an early event in the development of osteoarthritis. Cationic CECT imaging capitalizes on the electrostatic attraction between CA4+ and GAGs; exposing the proportional relationship between the amount of GAGs present within and the amount of CA4+ that diffuses into the tissue. The amount of CA4+ that resides in the tissue is then quantified through CECT imaging and estimates tissue integrity through nondestructive assessment.