Authorbridge Master Numbered Template A4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effectiveness of Distal Tibial Osteotomy
Nozaka et al. BMC Musculoskeletal Disorders (2020) 21:31 https://doi.org/10.1186/s12891-020-3061-7 RESEARCH ARTICLE Open Access Effectiveness of distal tibial osteotomy with distraction arthroplasty in varus ankle osteoarthritis Koji Nozaka* , Naohisa Miyakoshi, Takeshi Kashiwagura, Yuji Kasukawa, Hidetomo Saito, Hiroaki Kijima, Shuichi Chida, Hiroyuki Tsuchie and Yoichi Shimada Abstract Background: In highly active older individuals, end-stage ankle osteoarthritis has traditionally been treated using tibiotalar arthrodesis, which provides considerable pain relief. However, there is a loss of ankle joint movement and a risk of future arthrosis in the adjacent joints. Distraction arthroplasty is a simple method that allows joint cartilage repair; however, the results are currently mixed, with some reports showing improved pain scores and others showing no improvement. Distal tibial osteotomy (DTO) without fibular osteotomy is a type of joint preservation surgery that has garnered attention in recent years. However, to our knowledge, there are no reports on DTO with joint distraction using a circular external fixator. Therefore, the purpose of this study was to examine the effect of DTO with joint distraction using a circular external fixator for treating ankle osteoarthritis. Methods: A total of 21 patients with medial ankle arthritis were examined. Arthroscopic synovectomy and a microfracture procedure were performed, followed by angled osteotomy and correction of the distal tibia; the ankle joint was then stabilized after its condition improved. An external fixator was used in all patients, and joint distraction of approximately 5.8 mm was performed. All patients were allowed full weight-bearing walking immediately after surgery. Results: The anteroposterior and lateral mortise angle during weight-bearing, talar tilt angle, and anterior translation of the talus on ankle stress radiography were improved significantly (P < 0.05). -
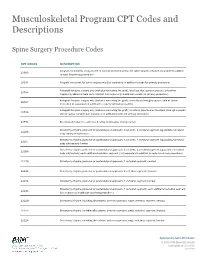
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Knee Joint Surgery: Open Synovectomy
Musculoskeletal Surgical Services: Open Surgical Procedures; Knee Joint Surgery: Open Synovectomy POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5588 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from HealthHelp. -

Radiation Synovectomy with 166Ho-Ferric Hydroxide: a First Experience
Radiation Synovectomy with 166Ho-Ferric Hydroxide: A First Experience Sedat Ofluoglu, MD1; Eva Schwameis, MD2; Harald Zehetgruber, MD2; Ernst Havlik, PhD3; Axel Wanivenhaus, MD2; Ingrid Schweeger, MD1; Konrad Weiss, MD4; Helmut Sinzinger, MD1; and Christian Pirich, MD1 1Department of Nuclear Medicine, University of Vienna, Vienna, Austria; 2Department of Orthopedics, University of Vienna, Vienna, Austria; 3Department of Biomedical Engineering and Physics and Ludwig Boltzmann Institute of Nuclear Medicine, Vienna, Austria; and 4Department of Nuclear Medicine, General Hospital of Wiener Neustadt, Wiener Neustadt, Austria lage, leading to the progressive loss of joint function and Radiation synovectomy (RS) is indicated when conventional significant disability. Treatment of chronic synovitis using pharmacologic treatment of chronic synovitis has not relieved radiation synovectomy (RS) aims to stop the inflammatory its symptoms. The use of radionuclides that are bound to ferric process causing pain, disability, and nonreversible structural hydroxide (FH) particles has been shown to be effective and damage to the joint (1–3). RS has been in clinical use for 166 safe for this procedure. Ho-FH macroaggregates offer prom- 50y(4) primarily as an alternative to surgical treatment (5). ising properties for RS but there is a lack of clinical data. We Safety is one of the most important aspects when radionu- investigated the efficacy and safety of 166Ho-FH in a prospective clinical trial in patients suffering from chronic synovitis. Meth- clides are applied therapeutically. The use of ferric hydrox- ods: Twenty-four intraarticular injections were performed in 22 ide (FH) particles as a carrier may offer some advantages patients receiving a mean activity of 1.11 GBq (range, 0.77–1.24 over other carriers with respect to the frequency and degree GBq) 166Ho-FH. -

ARTICULAR BLEEDING (HEMARTHROSIS) in HEMOPHILIA an ORTHOPEDIST’S POINT of VIEW Second Edition
TREATMENT OF HEMOPHILIA April 2008 · No. 23 ARTICULAR BLEEDING (HEMARTHROSIS) IN HEMOPHILIA AN ORTHOPEDIST’S POINT OF VIEW Second edition E. C. Rodríguez-Merchán Consultant Orthopedic Surgeon La Paz University Hospital Madrid, Spain Associate Professor of Orthopedics University Autonoma Madrid, Spain Published by the World Federation of Hemophilia (WFH), 2000; revised 2008. © Copyright World Federation of Hemophilia, 2008 The WFH encourages redistribution of its publications for educational purposes by not-for-profit hemophilia organizations. In order to obtain permission to reprint, redistribute, or translate this publication, please contact the Programs and Education Department at the address below. This publication is accessible from the World Federation of Hemophilia’s eLearning Platform at eLearning.wfh.org Additional copies are also available from the WFH at: World Federation of Hemophilia 1425 René Lévesque Boulevard West, Suite 1010 Montréal, Québec H3G 1T7 CANADA Tel. : (514) 875-7944 Fax : (514) 875-8916 E-mail: [email protected] Internet: www.wfh.org The Treatment of Hemophilia series is intended to provide general information on the treatment and management of hemophilia. The World Federation of Hemophilia does not engage in the practice of medicine and under no circumstances recommends particular treatment for specific individuals. Dose schedules and other treatment regimes are continually revised and new side-effects recognized. WFH makes no representation, express or implied, that drug doses or other treatment recommendations in this publication are correct. For these reasons it is strongly recommended that individuals seek the advice of a medical adviser and/or to consult printed instructions provided by the pharmaceutical company before administering any of the drugs referred to in this monograph. -

Advanced Imaging CPT Code List
Community Health Options: Advanced Imaging CPT Code List CPT® Category CPT® Code Description Code 3D Imaging 76376 3D Rendering W/O Postprocessing 3D Imaging 76377 3D Rendering W Postprocessing Cardiac Rhythm Myocardial strain imaging (quantitative assessment of myocardial mechanics using image-based analysis of local myocardial dynamics) Implantable 0399T (List separately in addition to code for primary procedure) Devices (CRID) Cardiac: CT 75571 Computed tomography, heart, without contrast material, with quantitative evaluation of coronary calcium Computed tomography, heart, with contrast material, for evaluation of cardiac structure and morphology (including 3d image Cardiac: CT 75572 postprocessing, assessment of cardiac function, and evaluation of venous structures, if performed) Computed tomography, heart, with contrast material, for evaluation of cardiac structure and morphology in the setting of congenital heart Cardiac: CT 75573 disease (including 3d image postprocessing, assessment of lv cardiac function, rv structure and function and evaluation of venous structures, if performed) Computed tomographic angiography, heart, coronary arteries and bypass grafts (when present), with contrast material, including 3d Cardiac: CT 75574 image postprocessing (including evaluation of cardiac structure and morphology, assessment of cardiac function, and evaluation of venous structures, if performed) Cardiac: RIGHT HEART CATHETERIZATION INCLUDING MEASUREMENT(S) OF OXYGEN SATURATION AND CARDIAC OUTPUT, WHEN Diagnostic Heart 93451 PERFORMED -
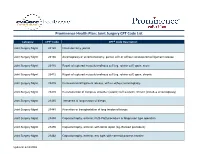
Master List of All CPT to Include Claims Only Codes for Any UM
Prominence Health Plan: Joint Surgery CPT Code List Category CPT® Code CPT® Code Description Joint Surgery Mgmt 23120 Claviculectomy; partial Joint Surgery Mgmt 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Surgery Mgmt 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Surgery Mgmt 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; chronic Joint Surgery Mgmt 23415 Coracoacromial ligament release, with or without acromioplasty Joint Surgery Mgmt 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Surgery Mgmt 23430 Tenodesis of long tendon of biceps Joint Surgery Mgmt 23440 Resection or transplantation of long tendon of biceps Joint Surgery Mgmt 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Surgery Mgmt 23455 Capsulorrhaphy, anterior; with labral repair (eg, Bankart procedure) Joint Surgery Mgmt 23462 Capsulorrhaphy, anterior, any type; with coracoid process transfer Updated: 6/12/2018 Category CPT® Code CPT® Code Description Joint Surgery Mgmt 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Surgery Mgmt 23470 Arthroplasty, glenohumeral joint; hemiarthroplasty Arthroplasty, glenohumeral joint; total shoulder (glenoid and proximal humeral replacement (eg, Joint Surgery Mgmt 23472 total shoulder)) Revision of total shoulder arthroplasty, including allograft when performed; humeral or glenoid Joint -

Should Synovectomy Be Performed in Primary Total Knee Arthroplasty For
Liu et al. Journal of Orthopaedic Surgery and Research (2019) 14:283 https://doi.org/10.1186/s13018-019-1332-5 RESEARCHARTICLE Open Access Should synovectomy be performed in primary total knee arthroplasty for osteoarthritis? A meta-analysis of randomized controlled trials Pei Liu1, Feifan Lu2, Jialiang Chen1, Ziqi Xia1, Huachen Yu3, Qidong Zhang4, Weiguo Wang4*† and Wanshou Guo4*† Abstract Background: During primary total knee arthroplasty (TKA), synovectomy as a part of the procedure has been recommended to relieve pain and inflammation of the synovium, but there is a controversy about it due to increased bleeding. In this meta-analysis, the aim is to answer whether synovectomy should be performed routinely during TKA for symptomatic knee osteoarthritis (KOA). Methods: Relevant randomized controlled trials (RCTs) on synovectomy were retrieved through database searches of PubMed, Embase, Web of Science, and Cochrane Library up to February 2019. Studies that compared postoperative pain, clinical Knee Society Score (KSS), functional KSS, range of motion (ROM), drainage, pre- and postoperative hemoglobin difference, transfusion rate, operative time, and/or complications were included in the meta-analysis. Review Manager 5.3.0 was used for meta-analysis. Results: We included 5 RCTs with 542 knees. Pooled results indicated that the synovectomy group was associated with more blood loss via drainage (WMD = − 99.41, 95% CI − 153.75 to − 45.08, P = 0.0003) and pre- and postoperative hemoglobin difference (WMD = − 0.93, 95% CI − 1.33 to − 0.5, P < 0.00001), compared with the non- synovectomy group. No statistically significant differences were demonstrated between both groups in postoperative pain, clinical KSS, functional KSS, ROM, transfusion rate, or complications (P > 0.05). -

2019 & 2020 Facility Scoring Methodology for Cost & Quality
2019 & 2020 Facility Scoring Methodology for Cost & Quality Designations In keeping with its goal of providing members with high-quality, lower-cost health care, Blue Cross Blue Shield of North Carolina (BCBSNC) developed the following methodology to best evaluate the quality and efficiency of partner facilities. Based on evaluation results, BCBSNC will designate PPO network facilities as Low Cost High Quality, High Quality, or Not Designated (NOTE: Critical Access facilities will maintain their Critical Access designation). This document describes the methodology used by Blue Cross NC for the 2019 and 2020 Cost & Quality Designations and is provided for informational purposes only. Designation Overview BCBSNC will base its designations on quality and efficiency. Any facility that does not meet the quality criteria will not be designated. Any practice that meets the quality criteria will also be assessed on efficiency criteria to determine its final designation. Please note, the designation is made at the facility level and only includes In-network Facilities contracted with BCBSNC. The initial driver of eligibility is the ability to meet the quality standards set forth in BCBSNC’s Facility-level standards (described below). Facilities that are unable to meet the quality standard for the network are not designated. Facilities that qualify for designation based on their quality evaluation will then be evaluated against BCBSNC’s efficiency criteria (described below) to determine if the organization meets the standards for the Low Cost High Quality designation. Facilities that meet both the quality and efficiency standards are designated as Low Cost High Quality. Facilities that meet the quality standard, but do not attain the efficiency standard, are designated as High Quality. -

Hip Joint Surgery: Arthrotomy for Biopsy of the Sacroiliac Joint Or Hip Joint
Musculoskeletal Surgical Services: Hip Joint Surgery: Arthrotomy for biopsy of the sacroiliac joint or hip joint POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5629 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from -

Arthroscopic Removal of Loose Bodies in Synovial Chondromatosis of Shoulder Joint, Unusual Location of Rare Disease: a Case Report and Literature Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by eCommons@AKU eCommons@AKU Section of Orthopaedic Surgery Department of Surgery 2019 Arthroscopic removal of loose bodies in synovial chondromatosis of shoulder joint, unusual location of rare disease: A case report and literature review Hussain Wahab Aga Khan University, [email protected] Obada Hasan Aga Khan University, [email protected] Ahmed Habib Aga Khan University, [email protected] Naveed Baloch Aga Khan University, [email protected] Follow this and additional works at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_orthop Part of the Orthopedics Commons, and the Surgery Commons Recommended Citation Wahab, H., Hasan, O., Habib, A., Baloch, N. (2019). Arthroscopic removal of loose bodies in synovial chondromatosis of shoulder joint, unusual location of rare disease: A case report and literature review. Annals of Medicine and Surgery, 37, 25-29. Available at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_orthop/94 Annals of Medicine and Surgery 37 (2019) 25–29 Contents lists available at ScienceDirect Annals of Medicine and Surgery journal homepage: www.elsevier.com/locate/amsu Case report Arthroscopic removal of loose bodies in synovial chondromatosis of shoulder joint, unusual location of rare disease: A case report and literature T review ∗ Hussain Wahab , Obada Hasan, Ahmed Habib, Naveed Baloch Department of Orthopaedics, Aga Khan University Hospital AKUH, Karachi, Pakistan ARTICLE INFO ABSTRACT Keywords: Introduction: Synovial chondromatosis is a benign mono-articular arthropathy affecting synovial joints. It mostly Arthroscopic synovectomy affects knee joint, followed by hip, elbow and wrist and is rarely reported for shoulder joint.