Guidelines for the Diagnosis and Management of Vulval Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaginal and Vulvar Cancer 10.1136/Ijgc-2020-ESGO.178
Int J Gynecol Cancer: first published as 10.1136/ijgc-2020-ESGO.177 on 4 December 2020. Downloaded from Abstracts 520 LONG TERM FOLLOW UP AFTER DIAGNOSIS OF Introduction/Background Since the introduction of the S2K GESTATIONAL TROPHOBLASTIC DISEASE AWMF guideline-based sentinel node biopsy technique in uni- focal vulvar cancer (diameter of <4 cm) and unsuspicious Pedro Corvelo Freitas, Beatriz Mira, António Guimarães, Ana Opinião, Hugo Nunes, Ana Francisca Jorge, Fátima Vaz, António Moreira. Instituto Português de Oncologia de Lisboa groin lymph nodes, the morbidity rate of patients has signifi- Francisco Gentil cantly decreased in Germany. The groin recurrence rate after IFL is vary from 0% to 5.8%, in contrast to 2.3% (95% CI, 10.1136/ijgc-2020-ESGO.176 0.6% to 5%) in unifocal vulvar cancer vs 3% (95% CI, 1% to 6%) in multifocal vulvar cancer after SLNB only, as sug- Introduction/Background The spectrum of Gestational tropho- gested in the GRoningen INternational Study on Sentinel blastic disease (GTD) ranges from pre-malignant conditions of node in Vulvar cancer (GROINSS-V-I) in 2008. Current guide- complete (CHM) and partial (PHM) hydatidiform moles to the lines suggest that in cases of metastasis of unilateral sentinel malignant invasive mole, choriocarcinoma (CC) and very rare lymph node (SLN) biopsy (B), groin node dissection, namely placental site trophoblastic tumour/epithelioid trophoblastic inguinofemoral lymphadenectomy (IFL), should be performed tumour (PSTT/ETT). Gestational trophoblastic neoplasia (GTN) bilaterally. However, a publication by Woelber et al. in Ger- are highly responsive to chemotherapy (CT) and with appropri- many and and Nica et al. -

FGM in Canada
Compiled by Patricia Huston MD, MPH Scientific Communications International, Inc for the Federal Interdepartmental Working Group on FGM. Copies of this report are available from: Women's Health Bureau Health Canada [email protected] The Canadian Women's Health Network 203-419 Graham Avenue Winnipeg, Manitoba R3C 0M3 fax: (204)989-2355 The opinions expressed in this report are not necessarily those of the Government of Canada or any of the other organizations represented. Dedication This report is dedicated to all the women in the world who have undergone FGM and to all the people who are helping them live with and reverse this procedure. This report is part of the ongoing commitment of Canadians and the Government of Canada to stop this practice in Canada and to improve the health and well-being of affected women and their communities. Executive Summary Female genital mutilation (FGM), or the ritual excision of part or all of the external female genitalia, is an ancient cultural practice that occurs around the world today, especially in Africa. With recent immigration to Canada of peoples from Somalia, Ethiopia and Eritrea, Sudan and Nigeria, women who have undergone this practice are now increasingly living in Canada. It is firmly believed by the people who practise it, that FGM improves feminine hygiene, that it will help eliminate disease and it is thought to be the only way to preserve family honour, a girl's virginity and her marriageability. FGM has a number of important adverse health effects including risks of infection and excessive bleeding (often performed when a girl is pre-pubertal). -

Pembrolizumab in Vaginal and Vulvar Squamous Cell Carcinoma: a Case Series from a Phase II Basket Trial Jefrey A
www.nature.com/scientificreports OPEN Pembrolizumab in vaginal and vulvar squamous cell carcinoma: a case series from a phase II basket trial Jefrey A. How 1, Amir A. Jazaeri 1, Pamela T. Soliman1, Nicole D. Fleming1, Jing Gong2, Sarina A. Piha‑Paul2, Filip Janku 2, Bettzy Stephen 2 & Aung Naing 2* Vaginal and vulvar squamous cell carcinoma (SCC) are rare tumors that can be challenging to treat in the recurrent or metastatic setting. We present a case series of patients with vaginal or vulvar SCC who were treated with single‑agent pembrolizumab as part of a phase II basket clinical trial to evaluate efcacy and safety. Two cases of recurrent and metastatic vaginal SCC, with multiple prior lines of systemic chemotherapy and radiation, received pembrolizumab. One patient had signifcant reduction (81%) in target tumor lesions prior to treatment discontinuation at cycle 10 following confrmed progression of disease with new metastatic lesions (stable disease by irRECIST criteria). In contrast, the other patient with vaginal SCC discontinued treatment after cycle 3 due to disease progression. Both patients had PD‑L1 positive vaginal tumors and tolerated treatment well. One case of recurrent vulvar SCC with multiple surgical resections and prior progression on systemic carboplatin had a 30% reduction in her target tumor lesions following pembrolizumab treatment with a PD‑L1 positive tumor. Treatment was discontinued for grade 3 mucositis after cycle 5. Pembrolizumab may provide some clinical beneft to some patients with vaginal or vulvar SCC and is overall safe to utilize in this population. Future studies are needed to evaluate the efcacy of pembrolizumab in these rare tumor types and to identify predictive biomarkers of response. -

Core Curriculum for Surgical Technology Sixth Edition
Core Curriculum for Surgical Technology Sixth Edition Core Curriculum 6.indd 1 11/17/10 11:51 PM TABLE OF CONTENTS I. Healthcare sciences A. Anatomy and physiology 7 B. Pharmacology and anesthesia 37 C. Medical terminology 49 D. Microbiology 63 E. Pathophysiology 71 II. Technological sciences A. Electricity 85 B. Information technology 86 C. Robotics 88 III. Patient care concepts A. Biopsychosocial needs of the patient 91 B. Death and dying 92 IV. Surgical technology A. Preoperative 1. Non-sterile a. Attire 97 b. Preoperative physical preparation of the patient 98 c. tneitaP noitacifitnedi 99 d. Transportation 100 e. Review of the chart 101 f. Surgical consent 102 g. refsnarT 104 h. Positioning 105 i. Urinary catheterization 106 j. Skin preparation 108 k. Equipment 110 l. Instrumentation 112 2. Sterile a. Asepsis and sterile technique 113 b. Hand hygiene and surgical scrub 115 c. Gowning and gloving 116 d. Surgical counts 117 e. Draping 118 B. Intraoperative: Sterile 1. Specimen care 119 2. Abdominal incisions 121 3. Hemostasis 122 4. Exposure 123 5. Catheters and drains 124 6. Wound closure 128 7. Surgical dressings 137 8. Wound healing 140 1 c. Light regulation d. Photoreceptors e. Macula lutea f. Fovea centralis g. Optic disc h. Brain pathways C. Ear 1. Anatomy a. External ear (1) Auricle (pinna) (2) Tragus b. Middle ear (1) Ossicles (a) Malleus (b) Incus (c) Stapes (2) Oval window (3) Round window (4) Mastoid sinus (5) Eustachian tube c. Internal ear (1) Labyrinth (2) Cochlea 2. Physiology of hearing a. Sound wave reception b. Bone conduction c. -
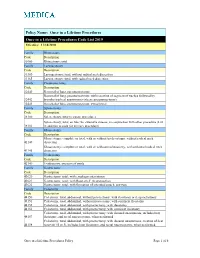
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Incidence and Cost of Anal, Penile, Vaginal and Vulvar Cancer in Denmark Jens Olsen1*, Tine Rikke Jørgensen2, Kristian Kofoed3 and Helle Kiellberg Larsen3
Olsen et al. BMC Public Health 2012, 12:1082 http://www.biomedcentral.com/1471-2458/12/1082 RESEARCH ARTICLE Open Access Incidence and cost of anal, penile, vaginal and vulvar cancer in Denmark Jens Olsen1*, Tine Rikke Jørgensen2, Kristian Kofoed3 and Helle Kiellberg Larsen3 Abstract Background: Besides being a causative agent for genital warts and cervical cancer, human papillomavirus (HPV) contributes to 40-85% of cases of anal, penile, vaginal and vulvar cancer and precancerous lesions. HPV types 16 & 18 in particular contribute to 74-93% of these cases. Overall the number of new cases of these four cancers may be relatively high implying notable health care cost to society. The aim of this study was to estimate the incidence and the health care sector costs of anal, penile, vaginal and vulvar cancer. Methods: New anogenital cancer patients were identified from the Danish National Cancer Register using ICD-10 diagnosis codes. Resource use in the health care sector was estimated for the year prior to diagnosis, and for the first, second and third years after diagnosis. Hospital resource use was defined in terms of registered hospital contacts, using DRG (Diagnosis Related Groups) and DAGS (Danish Outpatient Groups System) charges as cost estimates for inpatient and outpatient contacts, respectively. Health care consumption by cancer patients diagnosed in 2004–2007 was compared with that by an age- and sex-matched cohort without cancer. Hospital costs attributable to four anogenital cancers were estimated using regression analysis. Results: The annual incidence of anal cancer in Denmark is 1.9 per 100,000 persons. The corresponding incidence rates for penile, vaginal and vulvar cancer are 1.7, 0.9 and 3.6 per 100,000 males/females, respectively. -

Vaginal Cancer, Risk Factors, and Prevention Risk Factors for Vaginal
cancer.org | 1.800.227.2345 Vaginal Cancer, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors for vaginal cancer. ● Risk Factors for Vaginal Cancer ● What Causes Vaginal Cancer? Prevention There's no way to completely prevent cancer. But there are things you can do that might help lower your risk. Learn more here. ● Can Vaginal Cancer Be Prevented? Risk Factors for Vaginal Cancer A risk factor is anything that affects your chance of getting a disease such as cancer. Different cancers have different risk factors. Some risk factors, like smoking, can be changed. Others, like a person’s age or family history, can’t be changed. But having a risk factor, or even many, does not mean that you will get the disease. And 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 some people who get the disease may not have any known risk factors. Scientists have found that certain risk factors make a woman more likely to develop vaginal cancer. But many women with vaginal cancer don’t have any clear risk factors. And even if a woman with vaginal cancer has one or more risk factors, it’s impossible to know for sure how much that risk factor contributed to causing the cancer. Age Squamous cell cancer of the vagina occurs mainly in older women. It can happen at any age, but few cases are found in women younger than 40. Almost half of cases occur in women who are 70 years old or older. -

GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017
UnitedHealthcare® Commercial Medical Policy GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017 Table of Contents Page Related Commercial Policy INSTRUCTIONS FOR USE .......................................... 1 Blepharoplasty, Blepharoptosis and Brow Ptosis BENEFIT CONSIDERATIONS ...................................... 1 Repair COVERAGE RATIONALE ............................................. 2 Botulinum Toxin A and B DEFINITIONS .......................................................... 3 Cosmetic and Reconstructive Procedures APPLICABLE CODES ................................................. 4 DESCRIPTION OF SERVICES ...................................... 8 Gonadotropin Releasing Hormone Analogs CLINICAL EVIDENCE ................................................. 8 Panniculectomy and Body Contouring Procedures U.S. FOOD AND DRUG ADMINISTRATION ................... 11 Rhinoplasty and Other Nasal Surgeries CENTERS FOR MEDICARE AND MEDICAID SERVICES ... 11 Speech Language Pathology Services REFERENCES .......................................................... 11 POLICY HISTORY/REVISION INFORMATION ................ 12 Community Plan Policy Gender Dysphoria Treatment Related Optum Guideline Gender Dysphoria INSTRUCTIONS FOR USE This Medical Policy provides assistance in interpreting UnitedHealthcare benefit plans. When deciding coverage, the member specific benefit plan document must be referenced. The terms of the member specific benefit plan document [e.g., Certificate of Coverage (COC), Schedule of -

April 2013, Volume 5, Issue 4
VOLUME 5 MONTHLY ISSUE MEDICAL STAFF 4 NEWSLETTER ProgressNotes April TORRANCE MEMORIAL MEDICAL CENTER 2013 this issue CPOE P.1 Doctor’s Day P.2 MEC Approvals P.3-4 Medical Staff Calendar P.5 New Providers P.6 Roster Updates P.6 What is it? CPOE Select Rewards is a tiered program geared to reward pro- CPOE viders as they expand their use of CPOE (Computerized Provider Order Entry). Computerized How are providers enrolled in the CPOE Select Rewards Provider Order Program? Entry Select Enrollment is automatic as you or your group begins CPOE. Rewards Program How does it work? Providers qualify for monthly raffles based on meeting or exceed- ing CPOE percentage goals for their assigned tier. When will winners be announced? Winners will be announced the second Monday of every month, beginning in April. CPOE Monthly Raffle includes several prizes including an i-Pad mini. Individualized program details will be sent out to providers. Please call CPOE hotline at x2763 (xCPOE) if you have any questions. Doctor’s Day Thank you to all the physicians who attended the Doctor’s Day Celebration at Torrance Memorial on Wednesday, March 27th. The event was a success with many physicians attending to enjoy a great meal, music and prizes. Congratulations to Dr. Lauren Nguyen and Dr. James Flores, the two lucky winners of the iPad mini and the thirty physicians who also won gift cards to Nordstroms, California Pizza Kitchen, HealthLinks, Starbucks and iTunes. Torrance Memorial appreciates our physicians’ dedication and commitment to the health of our community. Medical Executive Committee Approvals The following items were presented and actions were approved at the March 12, 2013 Medical Executive Committee meeting: A. -

Vulvar Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis
cancer.org | 1.800.227.2345 Vulvar Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis Finding cancer early -- when it's small and before it has spread -- often allows for more treatment options. Some early cancers may have signs and symptoms that can be noticed, but that's not always the case. ● Can Vulvar Cancer Be Found Early? ● Signs and Symptoms of Vulvar Cancers and Pre-Cancers ● Tests for Vulvar Cancer Stages and Outlook (Prognosis) After a cancer diagnosis, staging provides important information about the extent of cancer in the body and anticipated response to treatment. ● Vulvar Cancer Stages ● Survival Rates for Vulvar Cancer Questions to Ask About Vulvar Cancer Here are some questions you can ask your cancer care team to help you better understand your cancer diagnosis and treatment options. ● Questions to Ask Your Doctor About Vulvar Cancer 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 Can Vulvar Cancer Be Found Early? Having pelvic exams and knowing any signs and symptoms of vulvar cancer greatly improve the chances of early detection and successful treatment. If you have any of the problems discussed in Signs and Symptoms of Vulvar Cancers and Pre-Cancers, you should see a doctor. If the doctor finds anything abnormal during a pelvic examination, you may need more tests to figure out what is wrong. This may mean referral to a gynecologist (specialist in problems of the female genital system). Knowing what to look for can sometimes help with early detection, but it is even better not to wait until you notice symptoms. -

NCCN Guidelines for Penile Cancer from Version 1.2019 Include
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Penile Cancer Version 2.2019 — May 13, 2019 NCCN.org Continue Version 2.2019, 05/13/19 © 2019 National Comprehensive Cancer Network® (NCCN®), All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN. NCCN Guidelines Index NCCN Guidelines Version 2.2019 Table of Contents Penile Cancer Discussion *Thomas W. Flaig, MD †/Chair Harry W. Herr, MD ϖ Sumanta K. Pal, MD † University of Colorado Cancer Center Memorial Sloan Kettering Cancer Center City of Hope National Medical Center *Philippe E. Spiess, MD, MS ϖ/Vice Chair Christopher Hoimes, MD † Anthony Patterson, MD ϖ Moffitt Cancer Center Case Comprehensive Cancer Center/ St. Jude Children’s Research Hospital/ University Hospitals Seidman Cancer Center The University of Tennessee Neeraj Agarwal, MD ‡ † and Cleveland Clinic Taussig Cancer Institute Health Science Center Huntsman Cancer Institute at the University of Utah Brant A. Inman, MD, MSc ϖ Elizabeth R. Plimack, MD, MS † Duke Cancer Institute Fox Chase Cancer Center Rick Bangs, MBA Patient Advocate Masahito Jimbo, MD, PhD, MPH Þ Kamal S. Pohar, MD ϖ University of Michigan Rogel Cancer Center The Ohio State University Comprehensive Stephen A. Boorjian, MD ϖ Cancer Center - James Cancer Hospital Mayo Clinic Cancer Center A. Karim Kader, MD, PhD ϖ and Solove Research Institute UC San Diego Moores Cancer Center Mark K. Buyyounouski, MD, MS § Michael P. Porter, MD, MS ϖ Stanford Cancer Institute Subodh M. Lele, MD ≠ Fred Hutchinson Cancer Research Center/ Fred & Pamela Buffett Cancer Center Sam Chang, MD ¶ Seattle Cancer Care Alliance Vanderbilt-Ingram Cancer Center Joshua J. -

Prognostic Significance of HPV and P16 Status in Men Diagnosed with Penile Cancer: a Systematic Review and Meta-Analysis
Published OnlineFirst July 9, 2018; DOI: 10.1158/1055-9965.EPI-18-0322 Review Cancer Epidemiology, Biomarkers Prognostic Significance of HPV and p16 Status in & Prevention Men Diagnosed with Penile Cancer: A Systematic Review and Meta-analysis Freja Lærke Sand1, Christina Louise Rasmussen1, Marie Hoffmann Frederiksen2, Klaus Kaae Andersen2, and Susanne K. Kjaer1,3 Abstract It has been shown that human papillomavirus (HPV) of DSS, we included 649 men with penile cancer tested for and p16 status has prognostic value in some HPV-associ- HPV (27% were HPV-positive) and 404 men tested for p16 ated cancers. However, studies examining survival in men expression (47% were p16-positive). The pooled HRHPV of with penile cancer according to HPV or p16 status are often DSS was 0.61 [95% confidence interval (CI), 0.38–0.98], inconclusive, mainly because of small study populations. andthepooledHRp16 of DSS was 0.45 (95% CI, 0.30– The aim of this systematic review and meta-analysis was to 0.69). In conclusion, men with HPV or p16-positive penile examine the association between HPV DNA and p16 status cancer have a significantly more favorable DSS compared and survival in men diagnosed with penile cancer. Multi- with men with HPV or p16-negative penile cancer. These ple electronic databases were searched. Twenty studies findings point to the possible clinical value of HPV and were ultimately included and study-specific and pooled p16 testing when planning the most optimal management HRs of overall survival and disease-specific survival (DSS) and follow-up strategy. Cancer Epidemiol Biomarkers Prev; 27(10); 1– were calculated using a fixed effects model.