Johnson & Johnson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Learner Notification International Society for Heart & Lung
Learner Notification International Society for Heart & Lung Transplantation (ISHLT) 41st Annual Meeting & Scientific Sessions Virtual Experience April 24 – 28, 2021 Live Virtual Acknowledgement of Financial Commercial Support Abbott Medtronic United Therapeutics Acknowledgement of In-Kind Commercial Support No in-kind commercial support was received for this educational activity. Satisfactory Completion Learners must complete an evaluation form to receive a certificate of completion. Your chosen sessions must be attended in their entirety as partial credit of individual sessions is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement. Physicians (ACCME) The International Society for Heart and Lung Transplantation (ISHLT) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Credit Designation Statement - ISHLT designates this live virtual activity for a maximum of 32.00 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Accreditation Statement In support of improving patient care, this activity has been planned and implemented by Amedco LLC and ISHLT. Amedco LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. Nurses (ANCC) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 ANCC contact hours. Pharmacists (ACPE) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 knowledge-based CPE contact hours. -

From the Academy
FROM THE ACADEMY Joint American Academy of DermatologyeNational Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy Craig A. Elmets, MD (Co-Chair),a HenryW.Lim,MD,b Benjamin Stoff, MD, MA,c Cody Connor, MD,a Kelly M. Cordoro, MD,d Mark Lebwohl, MD,e AprilW.Armstrong,MD,MPH,f Dawn M. R. Davis, MD,g Boni E. Elewski, MD,a Joel M. Gelfand, MD, MSCE,h Kenneth B. Gordon, MD,i AliceB.Gottlieb,MD,PhD,j Daniel H. Kaplan, MD, PhD,k Arthur Kavanaugh, MD,l Matthew Kiselica, BA/BS,m Dario Kivelevitch, MD,n Neil J. Korman, MD, PhD,o Daniela Kroshinsky, MD, MPH,p Craig L. Leonardi, MD,q Jason Lichten, MD,m NehalN.Mehta,MD,MSCE,r Amy S. Paller, MD,s Sylvia L. Parra, MD,t Arun L. Pathy, MD,u Elizabeth A. Farley Prater, MD,v Reena N. Rupani, MD,e Michael Siegel, PhD,m BruceE.Strober,MD,PhD,w,x Emily B. Wong, MD,y Jashin J. Wu, MD,z Vidhya Hariharan, PhD,aa and Alan Menter, MD (Co-Chair)n Birmingham, Alabama; Detroit, Michigan; Atlanta, Georgia; San Francisco, Los Angeles, San Diego, and Irvine, California; New York, New York; Rochester, Minnesota; Philadelphia and Pittsburgh, Pennsylva- nia; Milwaukee, Wisconsin; Portland, Oregon; Dallas and San Antonio, Texas; Cleveland, Ohio; Boston, Massachusetts; St. Louis, Missouri; Bethesda, Maryland; Chicago and Rosemont, Illinois; Sumter, South Carolina; Centennial, Colorado; Oklahoma City, Oklahoma; Farmington, Connecticut; and Waterloo, Ontario, Canada Psoriasis is a chronic inflammatory disease involving multiple organ systems and affecting approximately 3.2% of the world’s population. -

Women in Business Awards Luncheon at the Hotel Irvine, Where Aston Martin Americas President Laura Schwab Delivered the Keynote Address
10.5.20 SR_WIB.qxp_Layout 1 10/2/20 12:14 PM Page 29 WOMEN IN BUSINESS NOMINEES START ON PAGE B-60 INSIDE 2019 WINNERS GO BIG IN IRVINE, LAND NEW PARTNERS, INVESTMENTS PAGE 30 PRESENTED BY DIAMOND SPONSOR PLATINUM SPONSORS GOLD SPONSOR SILVER SPONSORS 10.5.20 SR_WIB.qxp_Layout 1 10/2/20 1:36 PM Page 30 30 ORANGE COUNTY BUSINESS JOURNAL www.ocbj.com OCTOBER 5, 2020 Winning Execs Don’t Rest on Their Laurels $1B Cancer Center Underway; Military Wins; Spanish Drug Investment Orange County’s business community last year celebrated the Business Journal’s 25th annual Women in Business Awards luncheon at the Hotel Irvine, where Aston Martin Americas President Laura Schwab delivered the keynote address. The winners, selected from 200 nominees, have not been resting on their laurels, even in the era of the coronavirus. Here are updates on what the five winners have been doing. —Peter J. Brennan Avatar Partners City of Hope Shortly after Marlo Brooke won the Busi- (AR) quality assurance solution for the U.S. As president of the City of Hope Orange employees down from Duarte. Area univer- ness Journal’s award for co-founding Hunt- Navy for aircraft wiring maintenance for the County, Annette Walker is orchestrating a sities could partner with City of Hope. ington Beach-based Avatar Partners Inc., Naval Air Systems Command’s Boeing V- $1 billion project to build one of the biggest, While the larger campus near the Orange she was accepted into the Forbes Technol- 22 Osprey aircraft. and scientifically advanced, cancer research County Great Park is being built, Walker in ogy Council, an invitation-only community Then the Air Force is using Avatar’s solu- centers in the world. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Annual Report on Annual Reports 2016
ANNUAL REPORT ON ANNUAL REPORTS 2016 TOP 400 ANNUAL REPORTS WHO RANKS WHERE? 100 ANNUALS IN BRIEF BEST REPORTING PRACTICES Company Value > Report Value Annual Report on Annual Reports 2016 Contents Report rating scale 3 Top 400 annual reports 4 Who ranks where? 25 From ABB to ZTE 100 annuals in brief 58 From Abbott to Yamaha How important is the annual report today? 92 Views from Cecilia Ketels, Kellie Friery, Renee Carter, David Robinson, Kaevan Gazdar, Elena Moskvina, Thomas Rosenmayr, Rob Stangroom, Andrey Kozhevnikov, Ana Santamarina, Katie Holcomb, Ananda Jagoda Best practices on key report attibutes 100 Strategy, message, investor information, risks, style, online… How we make it 121 How is your report doing? The report scan 127 The report rating panel 128 Robert Berick, Susan Blesener, Renee Carter, Vero Escarmelle, Helena Fournial, Kaevan Gazdar, Mike Guillaume, Pradip Seth, Eva Wolosiuk Making reports pay off 133 e.com – ReportWatch 135 2 Report rating scale A+ ééééé First-rate A éééé(é) Excellent A- éééé Very good B+ ééé(é) Sound B ééé Average B- éé(é) Uneven C+ éé Common C é(é) Substandard C- é Poor D (é) Uncompetitive 3 Top 400 annual reports AkzoNobel (No. 1) Electrolux (No. 2 ) SCA (No. 3) Volvo (No. 4) 4 Report rank Company Country Report rating Compare 1 AKZONOBEL Netherlands A+ DUPONT 2 ELECTROLUX Sweden A+ WHIRLPOOL 3 SCA Sweden A+ KIMBERLY-CLARK 4 VOLVO Sweden A+ DAIMLER 5 POTASHCORP Canada A+ AGRIUM 6 ATLAS COPCO Sweden A SANDVIK 7 STORA ENSO Finland A UPM 8 BOLIDEN Sweden A GLENCORE 9 WIENERBERGER Austria A BORAL 10 -

Drug Information Center Highlights of FDA Activities
Drug Information Center Highlights of FDA Activities – 9/1/20 – 9/30/20 FDA Drug Safety Communications & Drug Information Updates: Efficacy & Safety Concerns for Atezolizumab in Combination with Paclitaxel 9/8/20 The FDA alerted health care professionals and patients that a clinical trial evaluating atezolizumab plus paclitaxel in patients with previously untreated inoperable locally advanced or metastatic triple negative breast cancer that the drug combination was not effective. The combination of atezolizumab with another paclitaxel formulation, paclitaxel protein‐bound, is currently approved for use in adult patients with metastatic triple negative breast cancer, but this continued approval may be contingent on the results of additional studies. Paclitaxel should NOT be used as a replacement for paclitaxel protein bound in clinical practice. Electronic Expanded Access Requests 9/23/20 The FDA announced that the Reagan‐Udall Foundation has launched Expanded Access eRequest, a tool to submit expanded access requests for individual patient expanded access for drugs and biologics in non‐emergency settings. The tool allows auto population of forms, uploading of relevant documents, links to resources for physicians, patients, and caregivers, and secure application submission to the FDA. Benzodiazepine Drug Class: Drug Safety Communication ‐ Boxed Warning Update 9/23/20 The FDA is requiring the Boxed Warning be updated for all benzodiazepines to address serious risks of abuse, addiction, physical dependence, and withdrawal across the medication class. Changes are also being incorporated in the Medication Guides, and other sections of the prescribing information including the Warnings and Precautions, Drug Abuse and Dependence, and Patient Counseling Information sections. Diphenhydramine (Benadryl): Serious Problems with High Doses 9/24/20 The FDA issued a warning that taking higher than recommended doses of the common OTC allergy medication diphenhydramine (Benadryl) can lead to serious heart problems, seizures, coma, or even death. -

Nobel Endeavors in Immunology Introducing Dr
SPRING 2012 A PUBLICATION OF SOUTHWESTERN MEDICAL FOUNDATION Nobel Endeavors in Immunology Introducing Dr. Bruce Beutler, UT Southwestern’s fifth Nobel Laureate, and the new Center for the Genetics of Host Defense Southwestern Medical Foundation Board of Trustees 2011-2012 Edward M. Ackerman Joe M. Haggar, III Richard R. Pollock Sara Melnick Albert Nancy S. Halbreich Caren H. Prothro The Heritage Society Rafael M. Anchia LaQuita C. Hall Carolyn Perot Rathjen OF SOUTHWESTERN MEDICAL FOUNDATION Table of Contents Charlotte Jones Anderson Paul W. Harris* Mike Rawlings table of contents Barry G. Andrews Linda W. Hart Jean W. Roach Joyce T. Alban Mr. and Mrs. Thomas E. McCullough Marilyn H. Augur Joe V. (Jody) Hawn, Jr. Linda Robuck Mr. and Mrs. James R. Alexander Christopher F. McGratty Robert D. Rogers Ralph W. Babb, Jr. Jess T. Hay Anonymous (11) Carmen Crews McCracken McMillan Editor Doris L. Bass Frederick B. Hegi, Jr. Catherine M. Rose George A. Atnip# Ferd C. and Carole W. Meyer Nobel Endeavors in Immunology Peter Beck Jeffrey M. Heller* Billy Rosenthal Marilyn Augur* William R. and Anne E. Montgomery Heidi Harris Cannella The threads of Dr. Bruce Beutler’s scientific 3 # Jill C. Bee Julie K. Hersh Lizzie Horchow Routman* Paul M. Bass* Kay Y. Moran career are inextricably woven into the fabric of W. Robert Beavers, M.D. Barbara and Robert Munford Gil J. Besing Thomas O. Hicks Robert B. Rowling* Creative Director UT Southwestern’s history. From intern to mid-career Drs. Paul R. and Robert H. Munger# Jan Hart Black Sally S. Hoglund Stephen H. -

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

Approved Prenatal Medications Pain Medications • Tylenol
Approved Prenatal Medications Pain Medications Tylenol (acetaminophen) for minor aches and pains, headaches. (Do not use: Aspirin, Motrin, Advil, Aleve, Ibuprofen.) Coughs/Colds Robitussin (Cough) Robitussin DM (non-productive cough) DO NOT USE TILL OVER 12 WEEKS Secrets and Vicks Throat Lozenges Mucinex Sore Throat Chloraseptic spray Saline Gargle Sucrets and Vicks Throat Lozenges Antihistamines/Allergies Zyrtec Claritin Benadryl Dimetapp Insomnia Benadryl Unison Hemorrhoids Preparation H Tucks Anusol Diarrhea Imodium (1-2 doses- if it persists please notify the office) BRAT diet (bananas, rice, applesauce, toast) Lice RID (only!) DO NOT USE Kwell Itching Benadryl Calamine or Caladryl Lotion Hydrocortisone cream Heartburn, Indigestion, Gas Tums Gas-X Mylanta Pepcid Maalox Zantac *DO NOT USE PEPTO BISMOL- it contains aspirin Decongestants Sudafed Robitussin CF- Only if over 12 weeks Tavist D Ocean Mist Nasal Spray (saline solutions) Nausea Small Frequent Meals Ginger Ale Vitamin B6 Sea Bands Yeast Infections Monistat Mycolog Gyne-lotrimin Toothache Orajel May see dentists, have cavity filled using Novocain or lidocaine, have x-rays with double lead shield, may have antibiotics in the Penicillin family (penicillin, amoxicillin) Sweetners- all should be consumed in moderation with water being consumed more frequently Nutrisweet (aspartame) Equal (aspartame) Splenda (sucralose) Sweet’n Low (saccharin) *note avoid aspartame if you have phenylketonuria (PKU) Constipation Colace Fibercon Citrucel Senokot Metamucil Milk of Magnesia Fiberall Miralax Eczema Hydrocortisone Cream Medications to AVOID Accurate Lithium Paxil Ciprofloxacin Tetracycline Coumadin Other Chemicals to AVOID Cigarettes Alcohol Recreational Drugs: marijuana, cocaine, ecstasy, heroin . -

Over-The-Counter (OTC) Medications Applies To: Tufts Health Ritogether and Tufts Health Together*
Over-the-Counter (OTC) Medications Applies to: Tufts Health RITogether and Tufts Health Together* As communicated in the November 1, 2018 Provider Update, the following changes are effective for fill dates on or after January 1, 2019. As a result of this change, some OTC medications will require prior authorization in certain circumstances as outlined below: Brand-Name OTC Medication Has a Covered Interchangeable Generic Version Available Afrin No Drip Advil capsule Advil tablet Advil PM tablet Afrin Nasal Spray Original nasal solution Afrin No Drip Aveeno Oatmeal Severe nasal Aleve tablet Baciguent ointment Benadryl capsule Bath Pak Treatment solution Benadryl Allergy Benadryl Allergy Benadryl Allergy Benadryl Extra Benefiber powder tablet capsule Liquid Strength cream Caltrate 600 +D Centrum Silver Betadine Swabstick Caltrate + D tablet Centrum liquid Plus Minerals tablet tablet Centrum Silver Centrum Ultra Men’s Children’s Advil Centrum tablet Cheracol-D syrup Adult 50+ tablet tablet suspension Children’s Benadryl Citracal Calcium + Children’s Benadryl Children’s Tylenol Chlor-Trimeton Allergy chewable D Slow Release Allergy liquid suspension syrup tablet tablet Citrucel Fiber Claritin-D 12 hour Claritin-D 24 hour Clear Cough Liquid Citrucel tablet Laxative powder tablet tablet PM Dimetapp DM Dex4 Fast Acting Dimetapp Cold and Colace capsule Conceptrol 4% gel Cough and Cold Glucose liquid Allergy elixir elixir Dristan nasal spray Dulcolax tablet D-Vi-Sol liquid Ecotrin tablet Evac powder Ex-Lax chewable Gas-X chewable Feosol tablet -

Johnson & Johnson Reports 2010 Second-Quarter Results
Johnson & Johnson Reports 2010 Second-Quarter Results: Sales of $15.3 Billion Increased 0.6% Versus 2009 Second-Quarter; EPS was $1.23 Excluding Special Items, 2010 Second-Quarter EPS was $1.21, an increase of 5.2%* NEW BRUNSWICK, N.J., July 20, 2010 /PRNewswire via COMTEX News Network/ -- Johnson & Johnson (NYSE: JNJ) today announced sales of $15.3 billion for the second quarter of 2010, an increase of 0.6% as compared to the second quarter of 2009. Operational results increased 0.1% and the positive impact of currency was 0.5%. Domestic sales declined 2.8%, while international sales increased 4.1%, reflecting operational growth of 3.0% and a positive currency impact of 1.1%. Net earnings and diluted earnings per share for the second quarter of 2010 were $3.4 billion and $1.23, respectively. Second- quarter 2010 net earnings included an after-tax gain of $67 million representing the net impact of litigation matters. Excluding this special item, net earnings for the current quarter were $3.4 billion and diluted earnings per share were $1.21, representing increases of 5.4% and 5.2%, respectively, as compared to the same period in 2009.* The Company updated its earnings guidance for full-year 2010 to $4.65 - $4.75 per share, which excludes the impact of special items. The Company's guidance now reflects the impact of the voluntary recalls announced earlier this year of certain over-the-counter medicines and the suspension of manufacturing at the McNeil Consumer Healthcare facility in Fort Washington, Pa., as well as unfavorable changes in foreign currency exchange rates. -
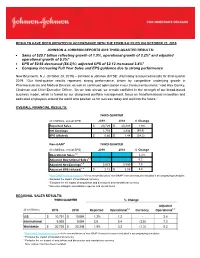
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures