From the Academy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Annual Report on Annual Reports 2016
ANNUAL REPORT ON ANNUAL REPORTS 2016 TOP 400 ANNUAL REPORTS WHO RANKS WHERE? 100 ANNUALS IN BRIEF BEST REPORTING PRACTICES Company Value > Report Value Annual Report on Annual Reports 2016 Contents Report rating scale 3 Top 400 annual reports 4 Who ranks where? 25 From ABB to ZTE 100 annuals in brief 58 From Abbott to Yamaha How important is the annual report today? 92 Views from Cecilia Ketels, Kellie Friery, Renee Carter, David Robinson, Kaevan Gazdar, Elena Moskvina, Thomas Rosenmayr, Rob Stangroom, Andrey Kozhevnikov, Ana Santamarina, Katie Holcomb, Ananda Jagoda Best practices on key report attibutes 100 Strategy, message, investor information, risks, style, online… How we make it 121 How is your report doing? The report scan 127 The report rating panel 128 Robert Berick, Susan Blesener, Renee Carter, Vero Escarmelle, Helena Fournial, Kaevan Gazdar, Mike Guillaume, Pradip Seth, Eva Wolosiuk Making reports pay off 133 e.com – ReportWatch 135 2 Report rating scale A+ ééééé First-rate A éééé(é) Excellent A- éééé Very good B+ ééé(é) Sound B ééé Average B- éé(é) Uneven C+ éé Common C é(é) Substandard C- é Poor D (é) Uncompetitive 3 Top 400 annual reports AkzoNobel (No. 1) Electrolux (No. 2 ) SCA (No. 3) Volvo (No. 4) 4 Report rank Company Country Report rating Compare 1 AKZONOBEL Netherlands A+ DUPONT 2 ELECTROLUX Sweden A+ WHIRLPOOL 3 SCA Sweden A+ KIMBERLY-CLARK 4 VOLVO Sweden A+ DAIMLER 5 POTASHCORP Canada A+ AGRIUM 6 ATLAS COPCO Sweden A SANDVIK 7 STORA ENSO Finland A UPM 8 BOLIDEN Sweden A GLENCORE 9 WIENERBERGER Austria A BORAL 10 -

Johnson & Johnson Has Made a New Breakthrough In
Investment idea: “Johnson & Johnson has made a new breakthrough in medicine! Shares are growing!” Johnson & Johnson (#JNJ) – is an American holding company that leads more than 250 subsidiaries worldwide, including: DePuy, Neutrogena, Johnson & Johnson Pharmaceutical Research and Development, Janssen Biotech, Alza, Cilag, Tibotec, Johnson & Johnson Consumer Companies and others. Johnson & Johnson is one of the world’s leading manufacturers of medical equipment, medicines and sanitary products. The total revenue of the company for 2018 amounted to $ 81.39 billion. Johnson & Johnson was founded in 1886. Factors indicating the purchase of JNJ shares in December: 1. Breakthrough developments in oncology. Johnson & Johnson is one of the leading companies in the field of oncology. So, the company Johnson & Johnson in November received positive reviews about the trials of a new drug from one of the kinds of cancer. It was immediately called a breakthrough. So far there is little news about this drug, but Johnson & Johnson shares are already strengthening on these information. 2. Seasonality and ascending dynamics of the DOW 30 index. Johnson & Johnson shares are prone to seasonal fluctuations, especially on the eve of the Christmas and New Year holidays. Cosmetology products and hygiene products of Johnson & Johnson are a classic gift in this period. The DOW 30 index, which includes JNJ shares, forms a reversal after a decline, which indicates a fundamental support. It is worth noting that the shares of Johnson & Johnson did not change significantly during the general decline in the stock index DOW 30. Fig. 1. DOW 30 index chart (blue line is JNJ shares) Johnson & Johnson shares are more likely to rise, despite the decline in the DOW30 index. -
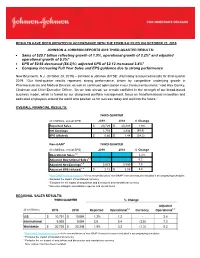
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

ASN Kidney Week 2020 Reimagined: Disclosures Page 1
10/14/2020 ASN Kidney Week 2020 Reimagined: Disclosures Page 1 Last Name First Name Nothing Employer Consultancy Ownership Interest Research Funding Honoraria Patents or Inventions Scientific Advisor or Membership Speakers Bureau Other Interests or Relationships to Disclose Abdel-Kader Khaled Vanderbilt University Medical Center BMC Nephrology; CJASN NKF Education committee; NIDDK Health IT work group Abudayyeh Ala University of Texas MD Anderson Cancer Center Adler Sharon Retrophin; Bristol Myers Squibb; Bayer; Retrophin; Bayer; ChemoCentryx; Omeros; Zyversa Bayer; Zyversa; Retrophin; AstraZeneca; Morphosys Retrophin; Bayer Pharmaceuticals; Zyversa; KDIGO; KRN; NephCure Kidney International AstraZeneca; ChemoCentryx; Omeros; Zyversa; Therapeutics; Calliditas; Morphosys AstraZeneca; Morphosys Foundation; Karger Publishers Morphosys; Karger Afkarian Maryam University of California, Davis Afrouzian Marjan University of Texas Medical Branch Alexion Pharmaceuticals; Banff Foundation Afshinnia Farsad Agarwal Anupam University of Alabama at Birmingham Dynamed - review content related to AKI for Goldilocks Therapeutics Genzyme/Sanofi Fabry Fellowship Award Univ Southern California, Vanderbilt, Emory, Akebia Editorial Board of AJP Renal, Kidney Int and My wife, Lisa Curtis, will be President-elect Dynamed and review updated materials prepared by Lab Investigation; invited to serve on for Women in Nephrology (2018-2019). Dynamed editorial team for AKI topics. Akebia - Advisory board of Goldilocks Therapeutics, Expert Panel to review new therapeutics -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Protagonist Therapeutics Names Samuel Saks, M.D., As Chief Development Officer
Protagonist Therapeutics Names Samuel Saks, M.D., as Chief Development Officer May 24, 2018 NEWARK, Calif., May 24, 2018 /PRNewswire/ -- Protagonist Therapeutics, Inc. (Nasdaq: PTGX) today announced the appointment of Samuel Saks, M.D., as Chief Development Officer. In this newly-created role, Dr. Saks will provide strategic oversight of the Company's research and clinical development programs. "We are very pleased to have Dr. Saks contribute his extensive industry experience to the company," commented Dinesh Patel, Chief Executive Officer of Protagonist Therapeutics. "His involvement will add to the depth of our research organization and assist in more effective clinical development of potentially transformative peptide-based drugs that have been discovered through our proprietary technology platform." Dr. Saks served as Chief Development Officer and board member at Auspex Pharmaceuticals, until the time of its acquisition by Teva Pharmaceuticals. Before his tenure at Auspex Pharmaceuticals, Dr. Saks was a co-founder of Jazz Pharmaceuticals, where he also served as Chief Executive Officer for six years. Earlier in his career, Dr. Saks held positions as company group chairman of ALZA Corporation and member of the Johnson & Johnson Pharmaceutical Operating Committee. Dr. Saks has also held leadership and management positions at Schering-Plough, Xoma and Genentech. Dr. Saks currently serves on the boards of directors of PDL BioPharma, TONIX Pharmaceuticals, Velocity Pharmaceutical Development and NuMedii. Dr. Saks received a B.S. in Biology and his M.D. from the University of Illinois. "Protagonist has a portfolio of well differentiated clinical development stage assets that present multiple choices to address unmet medical needs in diverse disease areas," added Dr. -
JJPAF PAH Brochure
We help financially eligible patients receive prescription medications donated by Johnson & Johnson operating companies. Visit www.JJPAF.org to learn more or call 833-919-3510 (toll free) 308-920-4358 (direct dial) If you don’t have prescription coverage and can’t get access to Johnson & Johnson operating companies’ medications, we may be able to help. The Patient Assistance Program Our free prescription program is available to anyone who meets the requirements listed below: • You have been prescribed a Johnson & Johnson donated medication • You meet the eligibility income requirements for the product(s) • You don’t have any insurance or your medication is not covered – Some patients with Medicare Prescription Drug Coverage (Part D) who cannot afford their medications and who meet certain financial criteria may also be eligible for assistance • You live in the United States or a U.S. territory • You are being treated by a U.S. licensed doctor as an outpatient How the program works About the Foundation Visit us online • The Patient Assistance Program covers five The Johnson & Johnson Patient Assistance Foundation, We invite you to navigate and explore pulmonary arterial hypertension (PAH) Inc. (JJPAF) is an independent, non-profit organization www.JJPAF.org on your computer, tablet, prescription products as well as over that is committed to helping eligible patients without or smart phone. Website features include: 35 other prescription medications insurance coverage receive prescription products • User-friendly navigation donated by Johnson & Johnson operating companies. – The list of currently available PAH • Medication search functionality medications can be found at www.JJPAF.org Last year, we distributed over 580,000 units of • Step-by-step interactive process to check medication, helping more than 95,000 people receive • Once you meet program requirements and are patient eligibility approved, you’ll receive your medications for the medications they need. -

Johnson & Johnson Annonce Que L'exécution De L'offre Publique D
Press Contacts: Amy Jo Meyer Johnson & Johnson annonce que l'Exécution de l'Offre Publique +1 (732) 524 6678 d'Acquisition visant Actelion est prévue pour le 16 juin 2017 [email protected] Annonce avoir reçu toutes les autorisations réglementaires nécessaires à Ernie Knewitz +1 (917) 697-2318 l'exécution de l'acquisition d'Actelion [email protected] Investor Contacts: NEW BRUNSWICK, N.J. – 9 juin 2017 – Johnson & Johnson (NYSE:JNJ) a Joseph J. Wolk annoncé aujourd'hui qu'avec la réception, ce jour, de l'approbation de l'acquisition +1 (732) 524-1142 projetée visant Actelion Ltd (SIX : ATLN) par la Commission européenne (CE), toutes les autorisations réglementaires nécessaires à l'exécution de la transaction Lesley Fishman +1 (732) 524-3922 ont été reçues. Johnson & Johnson s'attend à ce que l'exécution de l'offre publique d'acquisition entièrement en espèces de sa filiale suisse Janssen Holding GmbH, portant sur toutes les actions en mains du public d'Actelion Ltd au prix de $280 par action, payable en dollars américains, ait lieu le 16 juin 2017. Comme annoncé précédemment, dans le cadre de la transaction, Actelion transférera ses activités en matière de recherche médicamenteuse et ses actifs relatifs au développement préclinique dans une société biopharmaceutique suisse nouvellement constituée ("Idorsia Ltd"). Il est prévu que les actions d'Idorsia Ltd soient distribuées aux actionnaires d'Actelion au moyen d'un dividende en nature et qu'elles soient cotées à la SIX Swiss Exchange le jour de l'exécution de l'offre publique d'acquisition. Une filiale de Johnson & Johnson détiendra initialement 9.9 pour cent des actions d'Idorsia Ltd et elle dispose du droit d'éventuellement augmenter sa participation jusqu'à concurrence de 32 pour cent au moyen d'une obligation convertible. -

Christine Feorino)
Year-End Appeal 2015 YOUR SUPPORT HELPS PUT YOUTH ON THE RIGHT PATH Somerset Home’s mission is to aunt was not the best choice. provide abused, neglected, homeless, Fortunately however, he knew and runaway youth with housing, he could call Somerset Home for a stable environment, and help. Benji started in the Pathways supportive services to help program earlier this year. Pathways them become self-sufficient. counselors helped him get a steady Self-sufficiency is a key component job, learn how to budget, and find to ensure youth are prepared to live his own place. He is now working and thrive on their own well beyond two jobs – in retail and food service - Somerset Home. Through the and has saved more than $2,000. Pathways independent-living skills program, youth receive the tools and Until last year, Pathways worked knowledge needed for success. One mostly with clients who lived at of those youth is Benji... the Somerset Home residential programs. Since then, the program Benji was a resident at Passages. branched out to serve clients His father was incarcerated and his throughout central New Jersey. mother had substance abuse issues. The expansion has resulted in a As a result, he and his siblings would steady increase in referrals. This Pathways clients receive tools and knowledge to succeed in life. go weeks being neglected until they year, Pathways has served almost were ultimately put in foster care. 60 clients - a tremendous increase After living at Passages for several from previous years. employment, pertinent documents, driver’s licenses, and more. months, he decided to leave and live “I cannot thank Pathways enough for all your services”, said Benji. -

HIGHLIGHTS of PRESCRIBING INFORMATION TRACLEER® (Bosentan) Tablets These Highlights Do Not Include All the Information Needed to Use TRACLEER Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION TRACLEER® (bosentan) tablets These highlights do not include all the information needed to use TRACLEER safely and effectively. See full prescribing information for TRACLEER. • Reduce the dose and closely monitor patients developing aminotransferase TRACLEER® (bosentan) tablets, for oral use elevations more than 3 X Upper Limit of Normal (ULN) (2.1). ® TRACLEER (bosentan) tablets for oral suspension --------------------------- DOSAGE FORMS AND STRENGTHS --------------------------- Initial U.S. Approval: 2001 • Film-coated tablet: 62.5 mg and 125 mg (3) WARNING: RISKS OF HEPATOTOXICITY and EMBRYO-FETAL TOXICITY • Tablet for oral suspension: 32 mg (3) See full prescribing information for complete boxed warning. ----------------------------------- CONTRAINDICATIONS ----------------------------------- TRACLEER is available only through a restricted distribution program called • Pregnancy (4.1) the Bosentan REMS Program because of these risks (5.3): • Use with Cyclosporine A (4.2) Elevations of liver aminotransferases (ALT, AST) and liver failure have been • Use with Glyburide (4.3) reported with TRACLEER (5.1). • Hypersensitivity (4.4) • Measure liver aminotransferases prior to initiation of treatment and then -----------------------------WARNINGS AND PRECAUTIONS ----------------------------- monthly (2.1, 5.1). • Fluid retention: May require intervention (5.4). • Discontinue TRACLEER if aminotransferase elevations are accompanied by • Pulmonary veno-occlusive disease (PVOD): If signs of pulmonary edema signs or symptoms of liver dysfunction or injury or increases in bilirubin occur, consider the diagnosis of associated PVOD and consider discontinuing ≥2 x ULN (2.4, 5.1). TRACLEER (5.5). Based on animal data, TRACLEER is likely to cause major birth defects if used • Decreased sperm counts (5.6). during pregnancy (4.1, 5.2, 8.1). • Decreases in hemoglobin and hematocrit: Monitor hemoglobin levels after • Must exclude pregnancy before and during treatment (2.1, 4.1, 8.1).