A Solid-Phase Radioimmunoassay for The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Syllabus for M
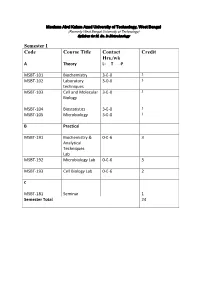
Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology Semester I Code Course Title Contact Credit Hrs./wk A Theory L- T -P MSBT-101 Biochemistry 3-0-0 3 MSBT-102 Laboratory 3-0-0 3 techniques MSBT-103 Cell and Molecular 3-0-0 3 Biology MSBT-104 Biostatistics 3-0-0 3 MSBT-105 Microbiology 3-0-0 3 B Practical MSBT-191 Biochemistry & 0-0-6 3 Analytical Techniques Lab MSBT-192 Microbiology Lab 0-0-6 3 MSBT-193 Cell Biology Lab 0-0-6 2 C MSBT-181 Seminar 1 Semester Total 24 Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology MSBT101: Biochemistry credits 3 Unit 1: Basic chemistry for biologists Formation of chemical bonds, molecular orbital (MO) theory and linear combination of atomic orbitals (LCAO),basics of mass spectrometry, molecules, Avogadro number, molarity, chemical reactions, reaction stoichiometry, rates of reaction, rate constants, order of reactions,kinetic versus thermodynamic controls of a reaction, reaction equilibrium (equilibrium constant); light and matter interactions (optical spectroscopy, fluorescence, bioluminescence, paramagnetism and diamagnetism, photoelectron spectroscopy; chemical bonds (ionic, covalent, Van derWalls forces); electronegativity, polarity; VSEPR theory and molecular geometry, dipole moment, orbital hybridizations; acids, bases and pH - Arrhenious theory, pH, ionic product of water, weak acids and bases, conjugate acid-base pairs, buffers and buffering action etc; chemical thermodynamics - internal energy, heat and temperature, enthalpy (bond enthalpy and reaction enthalpy), entropy, Gibbs free energy of ATP driven reactions, spontaneity versus driven reactions in biology;bond rotations and molecular conformations - Newman projections, conformational analysis of alkanes, alkenes and alkynes; functional groups, optically asymmetric carbon centers, amino acids, proteins, rotational freedoms in polypeptide backbone (Ramachandran plot). -

Technical Methods
J Clin Pathol 1987;40:581-588 J Clin Pathol: first published as 10.1136/jcp.40.5.581 on 1 May 1987. Downloaded from 56°C for 30 minutes. Technical methods Complement fixation tests were performed accord- ing to established methods,10 1 except that microtitre plates were used instead of World Health Organisation trays. For maximum sensitivity an ini- Cytomegalovirus (CMV) tial serum dilution of 1/4 was used. The antigen prep- antibody screening in blood aration used was a CMV complement fixation test antigen supplied by either Flow Laboratories Ltd, donors: modification of new latex Irvine, Scotland, or the Central Public Health Labo- ratory, Colindale, England. Guinea pig complements agglutination test compared with were supplied by Wellcome Diagnostics, Dartford, two standard methods England, or Don Whitly Scientific Ltd, Shipley, England. Complement fixation tests were performed A PUCKETT J E DAVIS From the Regional Blood using the following CMV antigen and complement Transfusion Centre, John Radeliffe Hospital, combinations: (1) PHLS CMV antigen + Wellcome Headington, Oxford, England Diagnostics complement, (2) PHLS CMV antigen + Don Whitly complement, and (3) Flow Laboratories CMV antigen + Wellcome Diagnostics complement. Infection with cytomegalovirus (CMV) is common, Immunofluorescence tests were performed and between 50 and 100% of adults may show evi- according to a standard method12 13 using substrate dence of infection.1 The transmission of the virus by slides of CMV infected (Westwood strain) fibroblasts blood transfusion2 and, therefore, the need to screen the Oxford Public Health Laboratory. donations intended for at risk groups such as provided by immunocompromised patients34 and neonates5 -7 iS CMV Scan passive latex agglutination kits were now well established. -

Laboratory Techniques Used for Immunological Laboratory Methods
Laboratory techniques used for Immunological laboratory methods Dr. Tatiana Jones, MD, PhD NCC How to Make Serial Dilutions? Interpretation can be made differently depending on the nature of test. For example, if we need to figure out in what sample the concentration of the antibody or antigen is higher, we will go by TITER, which is the lowest serial dilution (let’s say that it is 1:32 in the picture on the left) that gives us positive result. This mean that even diluted 32 times sample is still capable of reacting. The other scenario when we are interpreting quantitative assays, such as ELISA. In this case we need to match results of our samples to known concentrations of STANDARD and MULTIPLY be our dilution factor. What is Antibody Titer? An antibody titer is a measurement of how much antibody an organism has produced that recognizes a particular antigen. Titer is expressed as the inverse of the greatest dilution that still gives a positive result. ELISA is a common means of determining antibody titers. How to Determine Antibody Titer? Where we can use Indirect Coombs test detects the presence of anti-Rh antibodies in blood serum. A patient might be reported to have an "indirect Antibody Titer? Coombs titer" of 16. This means that the patient's serum gives a positive indirect Coombs test at any dilution down to 1/16 (1 part serum to 15 parts diluent). At greater dilutions the indirect Coombs test is negative. If a few weeks later the same patient had an indirect Coombs titer of 32 (1/32 dilution which is 1 part serum to 31 parts diluent), this would mean that more anti-Rh antibody was made, since it took a greater dilution to eradicate the positive test. -

Radial Immunodiffusion Assay Protocol
Radial Immunodiffusion Aim: To study the immunodiffusion technique by Single Radial Immunodiffusion. Introduction: Single Radial Immunodiffusion, also known as Mancini technique, is a quantitative immunodiffusion technique used to detect the concentration of antigen by measuring the diameter of the precipitin ring formed by the interaction of the antigen and the antibody at optimal concentration. In this method the antibody is incorporated into the agarose gel whereas the antigen diffuses into it in a radial pattern. Thus, the antibody is uniformly distributed throughout the gel. Principle: Single Radial Immunodiffusion is used extensively for the quantitative estimation of antigen. Here the antigen-antibody reaction is made more sensitive by the addition of antiserum into the agarose gel and loading the antigen sample in the well. As the antigen diffuses into the agarose radially in all directions, it’s concentration continuously falls until the equivalence point is reached at which the antigen concentration is in equal proportion to that of the antibody present in the agarose gel. At this point ring of precipitation (‘precipitin ring’) is formed around the well. The diameter of the precipitin ring is proportional to the concentration of antigen. With increasing concentration of antigen, precipitin rings with larger diameter are formed. The size of the precipitin rings depends on: Antigen concentration in the sample well Antibody concentration in the agarose gel Size of the sample well Volume of the sample Thus, by having various concentrations of a standard antigen, standard curve can be obtained from which one can determine the amount of an antigen in an unknown sample. Thus, this is a quantitative test. -

An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1981 An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate Allen H. Smith Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Biochemistry Commons Recommended Citation Smith, Allen H., "An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate" (1981). All Graduate Theses and Dissertations. 5295. https://digitalcommons.usu.edu/etd/5295 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. AN ENZYME- LINKED ThltviUNOSORBE!'IT ASSAY (ELISA) FOR PANTOTHENATE by Allen H. Smith A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biochemistry UTAH STATE UNIVERSITY Logan, Utah 1981 ii ACKNOWLEDGEMENTS To Dr. R. G. Hansen, to Dr. B. W. Wyse, to Carl Wittwer, to Jack Brown, to Jan Pearson, to Nedra Christensen, to all those who have made this experience one of tremendous growth, I express my thanks. I express appreciation to the United States Department of Agriculture, under Grant #5901-0410-9-0288-0 with Utah State University, for financial support. Finally, I express thanks to my parents, who have come to realize that graduate school is also a part of life. Allen H. Smith "iii TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ii LIST OF TABLES . vi LIST OF FIGURES. vii · ABSTRACT .. ix INTRODUCTION 1 REVIEW OF LITERATURE 4 Pantothenate Assays . -

Detection of Virus-Specific Immunoglobulins Using a Doubly Labeled Fluorescein- 125I Antibody A
JOURNAL OF CLINICAL MICROBIOLOGY, June 1976, p. 637-639 Vol. 3, No. 6 Copyright © 1976 American Society for Microbiology Printed in U.S.A. Detection of Virus-Specific Immunoglobulins Using a Doubly Labeled Fluorescein- 125I Antibody A. J. PARKINSON* AND J. KALMAKOFF Department ofMicrobiology, University of Otago, Dunedin, New Zealand Received for publication 17 February 1976 Commercially prepared fluorescein-labeled antihuman antibodies were la- beled with 125I and used to compare specific herpes simplex virus antibody titers as determined by indirect fluorescent antibody and radioimmunoassay tech- niques. Total virus-specific immunoglobulin and virus-specific immunoglobulin G titers did not vary by more than one twofold dilution when compared by the two methods. Efforts are being made to develop a reliable calf serum, penicillin (100 U/ml) streptomycin radioimmunoassay (RIA) for the detection of (100 ,ug/ml), and 0.1% bicarbonate, were in- virus-specific immunoglobulins, acceptable for fected with the isolated virus. Uninoculated use in diagnostic serology (1, 2, 5-8). The estab- monolayers were maintained as controls. When lishment of a satisfactory RIA depends on the infected monolayers showed 75% cytopathic use of antibody with both a high avidity and effect, both inoculated and uninoculated cells selectivity for the material to be assayed (4). were dispersed, using 0.015% ethylenediamine- Consequently, a major obstacle to using RIA tetraacetic acid. Both cellular suspensions were routinely is the necessity of preparing specific standardized to contain 2.5 x 105 cells/ml in high-titer antibody against human immuno- phosphate-buffered saline. Using 0.025-ml vol- globulins (IgG, IgM, and IgA). -

Importance of Ag-Ab Reactions
Ag-Ab reactions Tests for Ag-Ab reactions EISA SALEHI PhD. Immunology Dept. TUMS Importance of Ag-Ab Reactions • Understand the mechanisms of defense • Abs as tools in: – Treatment – Diagnosis • As biomarkers • As tools to measure analytes Nature of Ag/Ab Reactions http://www.med.sc.edu:85/chime2/lyso-abfr.htm • Lock and Key Concept • Non-covalent Bonds – Hydrogen bonds – Electrostatic bonds – Van der Waal forces – Hydrophobic bonds • Multiple Bonds • Reversible Source: Li, Y., Li, H., Smith-Gill, S. J., Mariuzza, R. A., Biochemistry 39, 6296, 2000 Affinity • Strength of the reaction between a single antigenic determinant and a single Ab combining site High Affinity Low Affinity Ab Ab Ag Ag Affinity = ( attractive and repulsive forces Calculation of Affinity Ag + Ab ↔ Ag-Ab Applying the Law of Mass Action: [[gAg-Ab] Keq = [Ag] x [Ab] Avidity • The overall strength of binding between an Ag with many determinants and multivalent Abs 4 6 10 Keq = 10 10 10 Affinity Avidity Avidity SifiitSpecificity • The ability of an individual antibody combining site to react with only one antigenic determinant. • The ability of a population of antibody molecules to react with only one antigen. Cross Reactivity • The ability of an individual Ab combining site to react with more than one antigenic determinant. • The ability of a population of Ab molecules to react with more than one Ag Cross reactions Anti-A Anti-A Anti-A Ab Ab Ab Ag A Ag B Ag C Shared epitope Similar epitope Factors Affecting Measurement of A/AbRAg/Ab Reac tions • Affinity • Avidity Ab excess Ag excess • AAbiAg:Ab ratio •Phyygsical form of Ag Equivalence – Lattice formation Do you need to know what happens in Lab. -

Making Antibodies Work
MILESTONES conjugated to the enzyme alkaline MILESTONE 4 phosphatase. They named their assay the ‘enzyme-linked immunosorbent assay’, which resulted in the catchy Making antibodies acronym ‘ELISA’. In addition to its application in the detection and work quantification of serum components, ELISAs are routinely used to detect viral infections, such as infection with human immunodeficiency virus, and the technique remains a mainstay of laboratories around the world. In addition to detecting the tagging of antibodies to molecules or cells of interest, it was clearly desira- ble to be able to separate the tagged components. This was achieved in Crossed immunoelectropho- 1979 by David Parks, Virginia Bryan, resis—just one analytical application of antibodies— Vernon Oi and Leonard Herzenberg, can simultaneously identify who used the newly invented dozens of serum proteins. Courtesy T.C.Bøg-Hansen. fluorescence-activated cell sorter. The light-scattering and fluorescent prop- erties of the cells enabled cells bound with antigen-coupled microspheres to be distinguished and directed Because antibodies are able to between the binding of antibodies into alternative collection pots, thus specifically bind target molecules, to endogenous insulin versus their facilitating phenotypic separation, the possibility of their having an binding to radioactive insulin. This monoclonal description and categorization. analytical application was recognized radioimmunoassay was used to antibodies… Since the pioneering work of early on. Robin Coombs, Arthur measure insulin present in the blood César Milstein and Georges J. F. Mourant and Robert Race, working and provided greater sensitivity than have had Köhler (MILESTONE 9) there have for the UK’s Medical Research that of previous approaches. -

Serological Methods in the Identification and Characterization of Viruses
CHAPTER 4 Serological Methods in the Identification and Characterization of Viruses M. H. V. Van Regenmortel Laboratoire de Virologie Institut de Biologie Mo!eculaire et Cellulaire 67000 Strasbourg, France 1. INTRODUCTION The purpose of this chapter is to present an integrated view of the various serological techniques that have been used in virology. The accent will be placed on the principles that govern each type of test and on the general applicability of the different serological techniques in all fields of virus research. In recent years, advances in serological tech niques have sometimes been applied in only one area of virology, although they could have been equally useful to workers studying other groups of viruses. No doubt this stems from the host-oriented approach that has guided the compartmentation of virology into separate fields of specialization. When it comes to serological properties, however, the similarities between animal, insect, bacterial, and plant viruses are paramount. The same immunochemical principles govern the in vitro serological reactions of all viral antigens, and much of general interest can be learned from the findings obtained with each particular group of viruses. An attempt will be made here to emphasize the general validity of specific experimental procedures. A number of recent reviews restricted to the serology of particular groups of viruses are available 183 H. Fraenkel-Conrat et al. (eds.), Comprehensive Virology © Plenum Press, New York 1981 184 Chapter 4 (Cowan, 1973; Schmidt and Lennette, 1973; Ball, 1974; Kurstak and Morisset, 1974; Burns and Allison, 1975; Mazzone and Tignor, 1976; Mayr et al., 1977; Tyrrell, 1978; Van Regenmortel, 1978; Cooper, 1979). -

Plasma and Cellular Retinoid-Binding Proteins and Transthyretin
Proc. Nati. Acad. Sci. USA Vol. 82, pp. 2488-2492, April 1985 Medical Sciences Plasma and cellular retinoid-binding proteins and transthyretin (prealbumin) are all localized in the islets of Langerhans in the rat (retlnoids/vitamin A/immunohistochemistry/radioimmunoassay) MICHIMASA KATO*t, KUNIYO KATO*t, WILLIAM S. BLANER*, BRUCE S. CHERTOWt, AND DEWITT S. GOODMAN*§ *Department of Medicine, Columbia University, College of Physicians and Surgeons, New York, NY 10032; and tDepartment of Medicine, Marshall University School of Medicine and Veterans Administration Medical Center, Huntington, WV 25701 Communicated by Seymour Lieperman, December 6, 1984 ABSTRACT The immunohistochemical localization of roles that CRBP and CRABP play within cells have not been plasmia retinol-binding protein' (RBP), cellular retinol-binding established, it has been suggested that these binding proteins protein (CRBP), and transtiyretin (TTR) was studied in rat may be involved in the biological expression of retinoid pancreas. The studies employed antibodies purified by im- activity within cells (see ref. 6 for a recent review). munosorbent affinity chromatography, permitting the specific We have' recently reported studies on the immunohis- staining and localization of each antigen by the, unlabeled tochemical localization of RBP, TTR, and CRBP in rat liver peroxidase-antiperoxidase method. Specific immunostaining and kidney (7). Each' of these proteins was found to be for each of these three proteins was found localized to the islets localized in specific cells within each organ. Highly specific ofLangerhans. Both RBPand CRBP were localized in cells that localization of CRBP has also been observed in the testis and were, peripherally distributed within the islets,'with an ana- epididymis (8, 9). -

MCB 407 – Immunology and Immunochemistry COURSE PARTICULARS COURSE INSTRUCTORS COURSE DESCRIPTION
D DEPARTMENT OF MICROBIOLOGY MCB 407 – Immunology and Immunochemistry COURSE PARTICULARS Course Code: MCB 407 Course Title: Immunology and Immunochemistry No. of Units: 4 Course Duration: Three hours of theory and three hours of practical per week for 15 weeks. Status: Compulsory Course Email Address: [email protected] Course Webpage: http://www.fwt.futa.edu.ng/courseschedule.php?coursecode=MCB%407 Prerequisite: BIO 201, BCH 201 COURSE INSTRUCTORS Professor (Mrs). T. T. Adebolu Microbiology Office Annex, Room 14 Dept. of Microbiology, Federal University of Technology, Akure, Nigeria. Phone: +2348053617571 Email: [email protected] and Dr. M. K. Oladunmoye Postgraduate Research Laboratory Phase 1, Dept. of Microbiology, Federal University of Technology, Akure, Nigeria. Phone: +2348035057977 Email: [email protected] COURSE DESCRIPTION Basic concept of Immunology. Antigens and antigenic determinants. Antibodies. Structures and classification of immunoglobulins/antibodies. Antigen and antibody reactions. Innate and Acquired Immunity. Immune response. Hypersensitivity reactions. Autoimmune diseases. Immunodeficiency diseases. Introduction to transplantation immunology. The practicals will include laboratory exercise in modern techniques in immunology and immunochemistry. 1 COURSE OBJECTIVES The objectives of this course are to: give the students an insight to the basic concept of immunology; expose the students to the major determinants that confer immunity in a host to infections; and acquire practical skills for immunodiagnosis -

Msc Biochemistry Autonomy 2015-17
Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad–500094 Autonomous (Accredited with ‘A’ grade by NAAC) MSc Biochemistry Autonomy 2015-17 Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad–500094 Autonomous (Accredited with ‘A’ grade by NAAC) M.Sc. Biochemistry Syllabus (Effective from 2015 admitted batch) SEMESTER I PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I : BI101T:Chemistry and Metabolism of Proteins, Lipids and Porphyrins 4 4 30 70 2 Paper-II : BI102T:Chemistry and Metabolism of Carbohydrates, Vitamins 4 4 30 70 and Nucleic Acids 3 Paper-III: BI 103T: Bio-Analytical Techniques 4 4 30 70 4 Paper-IV: BI104T:BioenergeticsAndCellBiology 4 4 30 70 5 Paper-V: BI105P: Amino acids and protein analysis 8 4 -- 100 6 Paper-VI: BI106P: Carbohydrate and lipid analysis 8 4 -- 100 Total 32 24 120 480 SEMESTER II PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I:BI201T:Enzymology 4 4 30 70 2 Paper-II:BI202T:MolecularBiology 4 4 30 70 3 Paper-III:BI203T:BiochemicalGeneticsAndModelOrganisms 4 4 30 70 4 Paper-IV: B1 204T: Computational methods and Cell study methods 4 4 30 70 5 Paper-V:BI205P:Enzymology and Biochemical preparations 8 4 -- 100 6 Paper-VI:BI206P: Molecular Biology, Genetics and Quantitative Biology 8 4 -- 100 Total 32 24 120 480 SEMESTER III PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I:BI301T:GeneRegulation and Genetic Engineering 4 4 30 70 2 Paper-II:BI302T:Immunology and Immunotechnology 4 4 30 70 3 Paper-III:BI303T: Virology, Nutrition & Clinical Biochemistry.