Serological Methods in the Identification and Characterization of Viruses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meningitis Manual Text
Laboratory Methods for the Diagnosis of MENINGITIS Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae Centers for Disease Control and Prevention August, 1998 Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae Table of Contents Introduction………………………………………………………………………………… 1 Acknowledgments ……………………………………………………………………….. 2 I. Epidemiology of Meningitis Caused by Neisseria meningitidis, Haemophilus influenzae and Streptococcus pneumoniae,…………………………………………… 3 II. General Considerations ......................................................................................................... 5 A. Record Keeping ................................................................................................................... 5 III. Collection and Transport of Clinical Specimens ................................................................... 6 A. Collection of Cerebrospinal Fluid (CSF)............................................................................... 6 A1. Lumbar Puncture ................................................................................................... 6 B. Collection of Blood .............................................................................................................. 7 B1. Precautions ............................................................................................................ 7 B2. Sensitivity of Blood Cultures ................................................................................ -
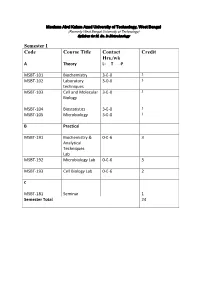
Syllabus for M
Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology Semester I Code Course Title Contact Credit Hrs./wk A Theory L- T -P MSBT-101 Biochemistry 3-0-0 3 MSBT-102 Laboratory 3-0-0 3 techniques MSBT-103 Cell and Molecular 3-0-0 3 Biology MSBT-104 Biostatistics 3-0-0 3 MSBT-105 Microbiology 3-0-0 3 B Practical MSBT-191 Biochemistry & 0-0-6 3 Analytical Techniques Lab MSBT-192 Microbiology Lab 0-0-6 3 MSBT-193 Cell Biology Lab 0-0-6 2 C MSBT-181 Seminar 1 Semester Total 24 Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology MSBT101: Biochemistry credits 3 Unit 1: Basic chemistry for biologists Formation of chemical bonds, molecular orbital (MO) theory and linear combination of atomic orbitals (LCAO),basics of mass spectrometry, molecules, Avogadro number, molarity, chemical reactions, reaction stoichiometry, rates of reaction, rate constants, order of reactions,kinetic versus thermodynamic controls of a reaction, reaction equilibrium (equilibrium constant); light and matter interactions (optical spectroscopy, fluorescence, bioluminescence, paramagnetism and diamagnetism, photoelectron spectroscopy; chemical bonds (ionic, covalent, Van derWalls forces); electronegativity, polarity; VSEPR theory and molecular geometry, dipole moment, orbital hybridizations; acids, bases and pH - Arrhenious theory, pH, ionic product of water, weak acids and bases, conjugate acid-base pairs, buffers and buffering action etc; chemical thermodynamics - internal energy, heat and temperature, enthalpy (bond enthalpy and reaction enthalpy), entropy, Gibbs free energy of ATP driven reactions, spontaneity versus driven reactions in biology;bond rotations and molecular conformations - Newman projections, conformational analysis of alkanes, alkenes and alkynes; functional groups, optically asymmetric carbon centers, amino acids, proteins, rotational freedoms in polypeptide backbone (Ramachandran plot). -
![SARS-Cov-2) (Coronavirus Disease [COVID-19]](https://docslib.b-cdn.net/cover/2980/sars-cov-2-coronavirus-disease-covid-19-182980.webp)
SARS-Cov-2) (Coronavirus Disease [COVID-19]
CPT® Category I and Proprietary Laboratory Analyses (PLA) Codes for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) Most recent changes to this medium descriptor document: • Addition of 8 Category I codes (0004A, 0051A, 0052A, 0053A, 0054A, 0064A, 91305, 91306) accepted by the CPT Editorial Panel. Codes 0004A, 0051A, 0052A, 0053A, 0054A, 0064A, 91305, 91306 and all related references will be published in CPT 2023. • Deleted codes in this document appear with a strikethrough. It is important to note that further CPT Editorial Panel or Executive Committee actions may affect these codes and/or descriptors. For this reason, code numbers and/or descriptor language in the CPT code set may differ at the time of publication. In addition, further Panel actions may result in gaps in code number sequencing. The following code was accepted at the March 2020 CPT Editorial Panel meeting for the 2021 CPT production cycle. This code is effective immediately on March 13, 2020. Released to Code Medium Code Descriptor Effective Publication AMA website ⚫87635 IADNA SARS-COV-2 COVID-19 AMPLIFIED PROBE TQ March 13, 2020 March 13, 2020 CPT® 2021 The following codes were accepted and revised at the April 2020 CPT Editorial Panel meeting for the 2021 CPT production cycle. These codes are effective immediately on April 10, 2020. IMMUNOASSAY INFECTIOUS AGT ANTIBODY 86318 QUAL/SEMIQUAN 1 STEP METH April 10, 2020 April 10, 2020 CPT® 2021 #⚫86328 IA INFECTIOUS AGT ANTIBODY SARS-COV-2 COVID-19 April 10, 2020 April 10, 2020 CPT® 2021 ⚫86769 ANTB SEVERE AQT RESPIR SYND SARS-COV-2 COVID- April 10, 2020 April 10, 2020 CPT® 2021 19 The following code was accepted by the Executive Committee of the CPT Editorial Panel. -

Master Seed Testing in the Virology Section
VIRSOP2007.04 Page 1 of 8 United States Department of Agriculture Center for Veterinary Biologics Standard Operating Policy/Procedure Master Seed Testing in the Virology Section Date: March 27, 2018 Number: VIRSOP2007.04 Supersedes: VIRSOP2007.03, October 10, 2014 Contact: Alethea M. Fry, (515) 337-7200 Sandra K. Conrad Peg A. Patterson Approvals: /s/Geetha B. Srinivas Date: 01Aug18 Geetha B. Srinivas, Section Leader Virology United States Department of Agriculture Animal and Plant Health Inspection Service P. O. Box 844 Ames, IA 50010 Mention of trademark or proprietary product does not constitute a guarantee or warranty of the product by USDA and does not imply its approval to the exclusion of other products that may be suitable. Entered into CVB Quality Management System by: /s/Linda S. Snavely 02Aug18 Linda S. Snavely Date Quality Management Program Assistant UNCONTROLLED COPY Center for Veterinary Biologics VIRSOP2007.04 Standard Operating Policy/Procedure Page 2 of 8 Master Seed Testing in the Virology Section Table of Contents 1. Purpose/Scope 2. Prerequisites for Master Seed Extraneous Agent Testing 2.1 Master Seed historical information 2.2 Substrate purity requirements 2.3 Testing of Master Seed consisting of persistently infected master cell stock 3. Testing Procedures for Detection of Extraneous Agents in Master Seeds 3.1 Neutralization of Master Seed 3.2 Passage of Master Seed in permissive cells or culture system 3.3 Detection of extraneous agents by fluorescent antibody (FA) staining 3.4 Detection of hemadsorbing agents 3.5 Detection of viral intracellular inclusions and related cellular changes by the hematoxylin and eosin staining method 3.6 Detection of Seneca Virus A (SVA) 4. -

Technical Methods
J Clin Pathol 1987;40:581-588 J Clin Pathol: first published as 10.1136/jcp.40.5.581 on 1 May 1987. Downloaded from 56°C for 30 minutes. Technical methods Complement fixation tests were performed accord- ing to established methods,10 1 except that microtitre plates were used instead of World Health Organisation trays. For maximum sensitivity an ini- Cytomegalovirus (CMV) tial serum dilution of 1/4 was used. The antigen prep- antibody screening in blood aration used was a CMV complement fixation test antigen supplied by either Flow Laboratories Ltd, donors: modification of new latex Irvine, Scotland, or the Central Public Health Labo- ratory, Colindale, England. Guinea pig complements agglutination test compared with were supplied by Wellcome Diagnostics, Dartford, two standard methods England, or Don Whitly Scientific Ltd, Shipley, England. Complement fixation tests were performed A PUCKETT J E DAVIS From the Regional Blood using the following CMV antigen and complement Transfusion Centre, John Radeliffe Hospital, combinations: (1) PHLS CMV antigen + Wellcome Headington, Oxford, England Diagnostics complement, (2) PHLS CMV antigen + Don Whitly complement, and (3) Flow Laboratories CMV antigen + Wellcome Diagnostics complement. Infection with cytomegalovirus (CMV) is common, Immunofluorescence tests were performed and between 50 and 100% of adults may show evi- according to a standard method12 13 using substrate dence of infection.1 The transmission of the virus by slides of CMV infected (Westwood strain) fibroblasts blood transfusion2 and, therefore, the need to screen the Oxford Public Health Laboratory. donations intended for at risk groups such as provided by immunocompromised patients34 and neonates5 -7 iS CMV Scan passive latex agglutination kits were now well established. -

Developmental Sequence and Intracellular Sites of Synthesis of Three Structural Protein Antigens of Influenza A2 Virus
JOURNAL OF VIROLOGY, Feb. 1970, p. 153-164 Vol. 5, No. 2 Copyright © 1970 American Society for Microbiology Printed in U.S.A. Developmental Sequence and Intracellular Sites of Synthesis of Three Structural Protein Antigens of Influenza A2 Virus KOICHIRO MAENO1 AND EDWIN D. KILBOURNE Department of Microbiology, Mount Sinai School of Medicine of The City U iversity of New York, New York, New York 10049 Received for publication 17 September 1969 Specific antisera for hemagglutinin (HA) and neuraminidase antigens of in- fluenza A2 virus (A2E) were produced through the segregation of the two pro- teins in reciprocal viral recombinants of A2E and Aoe viruses. Gamma globulin frac- tions of these specific antisera and of antiserum specific for the nucleoprotein (NP) antigen of Aoe virus were conjugated with fluorescein isothiocyanate and employed to follow the synthesis of the three structural proteins in clone 1-5C-4 human aneuploid cells, with parallel measurement of serological and biological activity of the antigens by other techniques. In this system, NP antigen appeared first (at 3 hr) in the cell nucleus, whereas HA and neuraminidase appeared coincidentally, at 4 hr after infection, in the cytoplasm. The initial detectability of biological or complement-fixing activity of the proteins coincided with their demonstrability as stainable antigens. Late in infection, all three antigens were detected at the cell surface. Antibody specific for HA partially blocked the intracellular staining of neuraminidase and inhibited the enzymatic activity of both extracted and intact extracellular virus. These observations suggest the close intracytoplasmic proximity of the two envelope antigens and perhaps their initial association in a larger protein. -

Polio Laboratory Manual
WHO/IVB/04.10 ORIGINAL: ENGLISH Polio laboratory manual 4th edition, 2004 The World Health Organization has managed The evaluation of the impact of vaccine- cooperation with its Member States and preventable diseases informs decisions to provided technical support in the fi eld of introduce new vaccines. Optimal strategies vaccine-preventable diseases since 1975. and activities for reducing morbidity and In 2003, the offi ce carrying out this function mortality through the use of vaccines are was renamed the WHO Department of implemented (Vaccine Assessment and Immunization, Vaccines and Biologicals. Monitoring). The Department’s goal is the achievement Efforts are directed towards reducing fi nancial of a world in which all people at risk are and technical barriers to the introduction protected against vaccine-preventable of new and established vaccines and diseases. Work towards this goal can be immunization-related technologies (Access to visualized as occurring along a continuum. Technologies). The range of activities spans from research, development and evaluation of vaccines Under the guidance of its Member States, to implementation and evaluation of WHO, in conjunction with outside world immunization programmes in countries. experts, develops and promotes policies and strategies to maximize the use and delivery WHO facilitates and coordinates research of vaccines of public health importance. and development on new vaccines and Countries are supported so that they immunization-related technologies for viral, acquire the technical and managerial skills, bacterial and parasitic diseases. Existing competence and infrastructure needed to life-saving vaccines are further improved and achieve disease control and/or elimination new vaccines targeted at public health crises, and eradication objectives (Expanded such as HIV/AIDS and SARS, are discovered Programme on Immunization). -

Detection of Influenza a Viruses from Environmental Lake and Pond Ice
TITLE “DETECTION OF INFLUENZA A VIRUSES FROM ENVIRONMENTAL LAKE AND POND ICE” Zeynep A. Koçer A Dissertation Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY August 2010 Committee: Scott O. Rogers, Advisor W. Robert Midden Graduate Faculty Representative John Castello George Bullerjahn Paul Morris ii ABSTRACT Scott O. Rogers, Advisor Environmental ice is an ideal matrix for the long-term protection of organisms due to the limitation of degradative processes. As a result of global climate change, some glaciers and polar ice fields are melting at rapid rates. This process releases viable microorganisms that have been embedded in the ice, sometimes for millions of years. We propose that viral pathogens have adapted to being entrapped in ice, such that they are capable of infecting naïve hosts after melting from the ice. Temporal gene flow, which has been termed genome recycling (Rogers et al., 2004), may allow pathogens to infect large host populations rapidly. Accordingly, we hypothesize that viable influenza A virions are preserved in lake and pond ice. Our main objective was to identify influenza A (H1-H16) from the ice of a few lakes and ponds in Ohio that have high numbers of migratory and local waterfowl visiting the sites. We developed a set of hemagglutinin subtype-specific primers for use in four multiplex RT-PCR reactions. Model studies were developed by seeding environmental lake water samples in vitro with influenza A viruses and subjecting the seeded water to five freeze-thaw cycles at -20oC and -80oC. -

COVID-19 Infection, Vaccines, and Immunity—The Antibody Response Requires Detailed Analysis
Opinion COVID-19 Infection, Vaccines, and Immunity—The Antibody Response Requires Detailed Analysis Nigel J. Dimmock School of Life Sciences, University of Warwick, Coventry CV4 7AL, UK; [email protected]; Tel.: +44-(0)7788728910 Abstract: Current tests for antibodies specific for the SARS-CoV-2 S protein say nothing about their precise epitope specificities. These data are needed to properly assess the immune status of individuals following infection or vaccination, and the risk posed by virus variants. Keywords: SARS-CoV-2; COVID-19; SARS-CoV-2 S protein; human neutralizing antibodies; epitope specificity; epitope mapping Assessment of immune responses to SARS-CoV-2, the virus responsible for COVID-19, is based almost entirely on the antibody response, and apart from clinical trials, compar- isons of the merits of one vaccine versus another, or of the first jab versus the second, or the immunity of individuals resulting from infection are all based on antibodies specific for the viral spike or S protein. Broadly speaking such antibodies are measured by a lateral flow test or similar in which antibody binds to the viral S protein, a test which is fast but non-quantitative and gives no indication of antibody function. Alternatively, there is the more labour-intensive neutralization test which measures the ability of antibody to inhibit Citation: Dimmock, N.J. virus infectivity in cell culture, under conditions that do not mimic the in vivo situation. COVID-19 Infection, Vaccines, and Both types of tests give information of limited value and say nothing about the epitope Immunity—The Antibody Response specificity of antibodies present. -

Diagnostic Potential of the Haemagglutination
Bulgarian Journal of Veterinary Medicine (2007), 10 , N o 3, 169 −178 DIAGNOSTIC POTENTIAL OF THE HAEMAGGLUTINATION INHIBITION TEST, THE IMMUNODIFFUSION TEST AND ELISA FOR DETECTION OF ANTIBODIES IN CHICKENS, INTRAVENOUSLY INFECTED WITH A/DUCK/BULGARIA/05 H6N2 AVIAN INFLUENZA VIRUS ISOLATE I. S. ZARKOV Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria Summary Zarkov, I., 2007. Diagnostic potential of the haemagglutination inhibition test, the immuno- diffusion test and ELISA for detection of antibodies in chickens, intravenously infected with A/duck/Bulgaria/05 H6N2 avian influenza virus isolate. Bulg. J. Vet. Med. , 10 , No 3, 169 −178. After experimental infection of chickens with an avian influenza viral isolate A/duck/Bulgaria/05 H6N2, the potential of the haemagglutination inhibition test (HIT), the immunodiffusion test (IDT) and enzyme-linked immunosorbent assay (ELISA) for antibody detection was evaluated. The results evidenced that in birds, haemagglutinins, precipitins and IgG antibodies, detectable with ELISA, were formed. The percentage of chickens with subtype-specific antibodies was the highest by the 21 st day of infection (100 %), followed by the 14 th (66.7 %) and the 28 th (55.6 %) days, and was the lowest by the 7 th day (44.4 %). Serum titres ranged between 1:4 and 1:256 with predomination of 1:8 and 1:16 titres (29.2 % each). The mean arithmetic titre for the experiment was 1:38.2. The highest percentage of chickens with precipitins was observed by the 14th day (55.6 %) followed by the 7 th , 21 st and the 28 th days with 33.3 % each. -

Research Article Isolation of a Reassortant H1N2 Swine Flu Strain of Type (Swine-Human-Avian) and Its Genetic Variability Analysis
Hindawi BioMed Research International Volume 2018, Article ID 1096079, 10 pages https://doi.org/10.1155/2018/1096079 Research Article Isolation of a Reassortant H1N2 Swine Flu Strain of Type (Swine-Human-Avian) and Its Genetic Variability Analysis Long-Bai Wang , Qiu-Yong Chen, Xue-Min Wu , Yong-Liang Che, Cheng-Yan Wang, Ru-Jing Chen, and Lun-Jiang Zhou Institute of Animal Husbandry and Veterinary Medicine, Fujian Academy of Agriculture Sciences, Fujian Animal Disease Control Technology Development Center, Fuzhou, Fujian 350013, China Correspondence should be addressed to Lun-Jiang Zhou; [email protected] Received 3 January 2018; Accepted 26 February 2018; Published 29 May 2018 Academic Editor: Jialiang Yang Copyright © 2018 Long-Bai Wang et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Weisolated an infuenza strain named A/Swine/Fujian/F1/2010 (H1N2) from a pig suspected to be infected with swine fu. Te results of electron microscopy, hemagglutination (HA) assay, hemagglutination inhibition (HI) assay, and whole genome sequencing analysis suggest that it was a reassortant virus of swine (H1N1 subtype), human (H3N2 subtype), and avian infuenza viruses. To further study the genetic evolution of A/Swine/Fujian/F1/2010 (H1N2), we cloned its whole genome fragments using RT-PCR and performed phylogenetic analysis on the eight genes. As a result, the nucleotide sequences of HA, NA, PB1, PA, PB2, NP, M, and NS gene are similar to those of A/Swine/Shanghai/1/2007(H1N2) with identity of 98.9%, 98.9%, 99.0%, 98.6%, 99.0%, 98.9%, 99.3%, and 99.3%, respectively. -

Immunoprophylaxis of Influenza Using AAV Vector Delivery of Cross
Immunoprophylaxis of Influenza using AAV Vector Delivery of Cross-Subtype Neutralizing Single Domain Antibodies JOANNE MARIE M. DEL ROSARIO Thesis submitted for the degree of Doctor of Philosophy Infection and Immunity University College London 2020 To Chris, as fate would have it. To Teki, thank you for everything. 2 DECLARATION I, Joanne Marie M. Del Rosario, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. __________________________ 3 ABSTRACT Cross-subtype neutralizing single domain antibodies against influenza present new opportunities for immunoprophylaxis and pandemic preparedness. Their simple modular structure and single open reading frame format are highly amenable to gene therapy-mediated delivery. R1a-B6, an alpaca-derived single domain antibody (nanobody), that is capable of potent cross-subtype neutralization in vitro of H1N1, H5N1, H2N2, and H9N2 influenza viruses, through binding to a highly conserved epitope in the influenza hemagglutinin stem region, was previously described. To evaluate the potential of R1a-B6 for immunoprophylaxis via adeno-associated viral (AAV) vector delivery, it was reformatted as Fc fusions of mouse IgG1 (ADCC-) and IgG2a (ADCC+) isotypes. This is also to extend R1a-B6’s half-life and to assess the requirement for ADCC for efficacy of R1a-B6 in vitro and in vivo. It was found that reformatted R1a-B6 of either mouse IgG isotype retained its potent binding and neutralization activity against different Group I influenza A subtypes in vitro. The findings in this study also demonstrate that a single intramuscular injection in mice of AAV encoding R1a-B6-Fc was able to drive sustained high-level expression (0.5–1.1 mg/mL) of the nanobody-Fc in sera with no evidence of reduction for up to 6 months.