Master Seed Testing in the Virology Section
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meningitis Manual Text
Laboratory Methods for the Diagnosis of MENINGITIS Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae Centers for Disease Control and Prevention August, 1998 Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae Table of Contents Introduction………………………………………………………………………………… 1 Acknowledgments ……………………………………………………………………….. 2 I. Epidemiology of Meningitis Caused by Neisseria meningitidis, Haemophilus influenzae and Streptococcus pneumoniae,…………………………………………… 3 II. General Considerations ......................................................................................................... 5 A. Record Keeping ................................................................................................................... 5 III. Collection and Transport of Clinical Specimens ................................................................... 6 A. Collection of Cerebrospinal Fluid (CSF)............................................................................... 6 A1. Lumbar Puncture ................................................................................................... 6 B. Collection of Blood .............................................................................................................. 7 B1. Precautions ............................................................................................................ 7 B2. Sensitivity of Blood Cultures ................................................................................ -
![SARS-Cov-2) (Coronavirus Disease [COVID-19]](https://docslib.b-cdn.net/cover/2980/sars-cov-2-coronavirus-disease-covid-19-182980.webp)
SARS-Cov-2) (Coronavirus Disease [COVID-19]
CPT® Category I and Proprietary Laboratory Analyses (PLA) Codes for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) Most recent changes to this medium descriptor document: • Addition of 8 Category I codes (0004A, 0051A, 0052A, 0053A, 0054A, 0064A, 91305, 91306) accepted by the CPT Editorial Panel. Codes 0004A, 0051A, 0052A, 0053A, 0054A, 0064A, 91305, 91306 and all related references will be published in CPT 2023. • Deleted codes in this document appear with a strikethrough. It is important to note that further CPT Editorial Panel or Executive Committee actions may affect these codes and/or descriptors. For this reason, code numbers and/or descriptor language in the CPT code set may differ at the time of publication. In addition, further Panel actions may result in gaps in code number sequencing. The following code was accepted at the March 2020 CPT Editorial Panel meeting for the 2021 CPT production cycle. This code is effective immediately on March 13, 2020. Released to Code Medium Code Descriptor Effective Publication AMA website ⚫87635 IADNA SARS-COV-2 COVID-19 AMPLIFIED PROBE TQ March 13, 2020 March 13, 2020 CPT® 2021 The following codes were accepted and revised at the April 2020 CPT Editorial Panel meeting for the 2021 CPT production cycle. These codes are effective immediately on April 10, 2020. IMMUNOASSAY INFECTIOUS AGT ANTIBODY 86318 QUAL/SEMIQUAN 1 STEP METH April 10, 2020 April 10, 2020 CPT® 2021 #⚫86328 IA INFECTIOUS AGT ANTIBODY SARS-COV-2 COVID-19 April 10, 2020 April 10, 2020 CPT® 2021 ⚫86769 ANTB SEVERE AQT RESPIR SYND SARS-COV-2 COVID- April 10, 2020 April 10, 2020 CPT® 2021 19 The following code was accepted by the Executive Committee of the CPT Editorial Panel. -

Developmental Sequence and Intracellular Sites of Synthesis of Three Structural Protein Antigens of Influenza A2 Virus
JOURNAL OF VIROLOGY, Feb. 1970, p. 153-164 Vol. 5, No. 2 Copyright © 1970 American Society for Microbiology Printed in U.S.A. Developmental Sequence and Intracellular Sites of Synthesis of Three Structural Protein Antigens of Influenza A2 Virus KOICHIRO MAENO1 AND EDWIN D. KILBOURNE Department of Microbiology, Mount Sinai School of Medicine of The City U iversity of New York, New York, New York 10049 Received for publication 17 September 1969 Specific antisera for hemagglutinin (HA) and neuraminidase antigens of in- fluenza A2 virus (A2E) were produced through the segregation of the two pro- teins in reciprocal viral recombinants of A2E and Aoe viruses. Gamma globulin frac- tions of these specific antisera and of antiserum specific for the nucleoprotein (NP) antigen of Aoe virus were conjugated with fluorescein isothiocyanate and employed to follow the synthesis of the three structural proteins in clone 1-5C-4 human aneuploid cells, with parallel measurement of serological and biological activity of the antigens by other techniques. In this system, NP antigen appeared first (at 3 hr) in the cell nucleus, whereas HA and neuraminidase appeared coincidentally, at 4 hr after infection, in the cytoplasm. The initial detectability of biological or complement-fixing activity of the proteins coincided with their demonstrability as stainable antigens. Late in infection, all three antigens were detected at the cell surface. Antibody specific for HA partially blocked the intracellular staining of neuraminidase and inhibited the enzymatic activity of both extracted and intact extracellular virus. These observations suggest the close intracytoplasmic proximity of the two envelope antigens and perhaps their initial association in a larger protein. -

Polio Laboratory Manual
WHO/IVB/04.10 ORIGINAL: ENGLISH Polio laboratory manual 4th edition, 2004 The World Health Organization has managed The evaluation of the impact of vaccine- cooperation with its Member States and preventable diseases informs decisions to provided technical support in the fi eld of introduce new vaccines. Optimal strategies vaccine-preventable diseases since 1975. and activities for reducing morbidity and In 2003, the offi ce carrying out this function mortality through the use of vaccines are was renamed the WHO Department of implemented (Vaccine Assessment and Immunization, Vaccines and Biologicals. Monitoring). The Department’s goal is the achievement Efforts are directed towards reducing fi nancial of a world in which all people at risk are and technical barriers to the introduction protected against vaccine-preventable of new and established vaccines and diseases. Work towards this goal can be immunization-related technologies (Access to visualized as occurring along a continuum. Technologies). The range of activities spans from research, development and evaluation of vaccines Under the guidance of its Member States, to implementation and evaluation of WHO, in conjunction with outside world immunization programmes in countries. experts, develops and promotes policies and strategies to maximize the use and delivery WHO facilitates and coordinates research of vaccines of public health importance. and development on new vaccines and Countries are supported so that they immunization-related technologies for viral, acquire the technical and managerial skills, bacterial and parasitic diseases. Existing competence and infrastructure needed to life-saving vaccines are further improved and achieve disease control and/or elimination new vaccines targeted at public health crises, and eradication objectives (Expanded such as HIV/AIDS and SARS, are discovered Programme on Immunization). -

COVID-19 Infection, Vaccines, and Immunity—The Antibody Response Requires Detailed Analysis
Opinion COVID-19 Infection, Vaccines, and Immunity—The Antibody Response Requires Detailed Analysis Nigel J. Dimmock School of Life Sciences, University of Warwick, Coventry CV4 7AL, UK; [email protected]; Tel.: +44-(0)7788728910 Abstract: Current tests for antibodies specific for the SARS-CoV-2 S protein say nothing about their precise epitope specificities. These data are needed to properly assess the immune status of individuals following infection or vaccination, and the risk posed by virus variants. Keywords: SARS-CoV-2; COVID-19; SARS-CoV-2 S protein; human neutralizing antibodies; epitope specificity; epitope mapping Assessment of immune responses to SARS-CoV-2, the virus responsible for COVID-19, is based almost entirely on the antibody response, and apart from clinical trials, compar- isons of the merits of one vaccine versus another, or of the first jab versus the second, or the immunity of individuals resulting from infection are all based on antibodies specific for the viral spike or S protein. Broadly speaking such antibodies are measured by a lateral flow test or similar in which antibody binds to the viral S protein, a test which is fast but non-quantitative and gives no indication of antibody function. Alternatively, there is the more labour-intensive neutralization test which measures the ability of antibody to inhibit Citation: Dimmock, N.J. virus infectivity in cell culture, under conditions that do not mimic the in vivo situation. COVID-19 Infection, Vaccines, and Both types of tests give information of limited value and say nothing about the epitope Immunity—The Antibody Response specificity of antibodies present. -

Serological Methods in the Identification and Characterization of Viruses
CHAPTER 4 Serological Methods in the Identification and Characterization of Viruses M. H. V. Van Regenmortel Laboratoire de Virologie Institut de Biologie Mo!eculaire et Cellulaire 67000 Strasbourg, France 1. INTRODUCTION The purpose of this chapter is to present an integrated view of the various serological techniques that have been used in virology. The accent will be placed on the principles that govern each type of test and on the general applicability of the different serological techniques in all fields of virus research. In recent years, advances in serological tech niques have sometimes been applied in only one area of virology, although they could have been equally useful to workers studying other groups of viruses. No doubt this stems from the host-oriented approach that has guided the compartmentation of virology into separate fields of specialization. When it comes to serological properties, however, the similarities between animal, insect, bacterial, and plant viruses are paramount. The same immunochemical principles govern the in vitro serological reactions of all viral antigens, and much of general interest can be learned from the findings obtained with each particular group of viruses. An attempt will be made here to emphasize the general validity of specific experimental procedures. A number of recent reviews restricted to the serology of particular groups of viruses are available 183 H. Fraenkel-Conrat et al. (eds.), Comprehensive Virology © Plenum Press, New York 1981 184 Chapter 4 (Cowan, 1973; Schmidt and Lennette, 1973; Ball, 1974; Kurstak and Morisset, 1974; Burns and Allison, 1975; Mazzone and Tignor, 1976; Mayr et al., 1977; Tyrrell, 1978; Van Regenmortel, 1978; Cooper, 1979). -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Studies on Influenzal Meningitis
STUDIES ON INFLUENZAL MENINGITIS 1. THE PROBLEMS O~ SPECIFIC THERAPY* BY HUGH K. WARD, M.B., AND JOYCE WRIGHT, B.M., B.Cm (From tke Department of Bacteriology and Immunology of tke Harvard University Medical Sckool, Boston) (Received for publication, November 9, 1931) INTRODUCTION Whatever the relation of Pfeiffer's Bacillus influenzae to epidemic in- fluenza-and that is not under discussion here there is no doubt that this organism is the cause of influenzal meningitis, a disease that is much more common than is generally realized. The records of The Children's Hospital, Boston, disclose that in the last 5 years there have been 56 cases of meningococcus meningitis and 25 cases of influenzal meningitis in children under 2 years of age. In both diseases the cases have occurred only sporadically during this period. Influenzal meningitis is a disease of infancy and early childhood and is almost always fatal. Rivers (1), in an analysis from the literature of 220 cases under 2 years of age, gives k mortality of 92 per cent. It is remarkable that no record can be found of any attempt being made to treat these cases with an appropriate antiserum, despite the striking results that have been obtained with antimeningococcus serum. Woll- stein (2) in 1911 injected antiserum intraspinally 24 hours after she had infected a rhesus monkey, the animal recovering, but no account can be found of the use of this serum in human cases. Rivers (3) has pointed out, and this has been our own experience, that the strains of the influenza bacillus that are found in influenzal meningitis are fairly homogeneous, thus simplifying the problem of specific serum treat- ment. -

USEPA MANUAL of METHODS for VIROLOGY - EPA Publication EPA/600/4-84/013 (R-12)
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx The following is an electronic text form of CHAPTER 12 of the USEPA MANUAL OF METHODS FOR VIROLOGY - EPA publication EPA/600/4-84/013 (R-12). The hardcopy form of this chapter can be obtained by contacting: Dr. Robert S. Safferman National Exposure Research Laboratory U.S. Environmental Protection Agency 26 West M.L. King Dr. Cincinnati OH 45268 USA e-mail: [email protected] Date of Publication (in Revised Form): May 1988 xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx CHAPTER 12 IDENTIFICATION OF ENTEROVIRUSES 1. INTRODUCTION A neutralization test used to identify enteroviruses is described in this chapter. The test utilizes reference-typing sera directed against isolated waterborne viruses. This renders the viruses noninfectious when treated with a matching serotype reagent. Virus inactivation is ascertained microscopically by observing the absence of host cell destruction in the presence of the virus-antibody mixture. The procedure consists of simultaneously inoculating virus and antiserum into a microtiter plate, incubating the virus-antibody mixture for 2 hr, adding a suspension of host cells to the mixture, incubating the host cells-virus-antibody mixture for three days, then examining the cells daily for five more days for the absence of cytopathic effects (CPE). The test utilizes the Lim Benyesh-Melnick (LB-M) antiserum pools which consist of 61 equine antisera. They include LB-M antiserum pools A-H for the identification of 41 enteroviruses, a single antiserum preparation for the identification of coxsackievirus B3 and LB-M pools J-P for the identification of 19 group A coxsackieviruses not identified by pools A-H. The A-H antiserum pools, prepared by Melnick and coworkers (1973), with instructions for rehydration and storage and with virus identification tables, had been previously available from the National Institutes of Health (NIH). -

LABORATORY TECHNIQUES in VIROLOGY by Marie P
LABORATORY TECHNIQUES IN VIROLOGY by Marie P. Fried Number 29 LABORATORY TECHNIQUES IN VIROLOGY by Marie P. Fried Number 29 Alaska Department of Fish and Game Division of Fisheries Rehabilitation, Enhancement and Development Don W. Collinsworth Commissioner Stanley A. Moberly Director P.O. Box 3-2000 Juneau, Alaska 99802 April 1984 TABLE OF CONTENTS PAGE Glossary' ........................................................... 1 Maintenance of stock cell lines: passage of confluent cell monolayer ................................................4 Incubating cell lines ..............................................6 Cytopathic effect of virus infection on tissue culture cells .........................................................7 Assays used in virology ............................................ 9 Collecting ovarian fluid, whole fish, and tissue samples for traditional assay ........................................ 10 Processing ovarian fluid, whole fish, and tissue samples for traditional assay ........................................ 13 Traditional assay .................................................15 Processing ovarian fluid, whole fish, and tissue samples for plaque assay .............................................19 Plaque assay ......................................................20 Serum neutralization assay ........................................25 Media .............................................................28 Detecting and avoiding bacterial and fungal contaminants ..........33 Washing glassware .................................................34 -

Pl Path 502 Plant Virus Serology
Plant Virus Serology Pl. Path. 502/602 PN Sharma Department of Plant Pathology CSK HPAU, Palampur-176062 (HP), INDIA Serology Based on antigen - antibody reaction Antigen and antibody reaction results in the formation of visible substrate, which is perhaps due to the formation of bridges between the two and thus form an aggregate which precipitate when big enough. Historical development Coons (1942), developed florescent antibody technique. Precipination in gels (Oudin, 1946; Ouchterolony, 1948). Modified agglutination test (Van Sloglerens, 1955). Double diffusion tests in plates (Octerlony, 1962) in tubes (Oakelay and Fullthrope, 1963). Bentonite flocculation test (Bozicevich et al., 1963). Kassamis (1972) reported potato mop top virus relatedness with TMV. Immuno-diffusion tests with Sodium dodecyl sulfate (SDS) (Purcifall and Batchelor, 1977). Modified latex test (Querfurth and & Paul 1979) Holling and Stone (1973) found groups of 18 viruses serologically related by immuno-diffusion and other tests. 1975 *Kohler and Milstein, Monoclonal antibodies used in genetic analysis Clark, M.F. (1975) – developed ELISA technique. Clark & Adam (1978) – used ELISA in Plants Virology. Blanksky and Derrick (1977) reported that seed borne viruses can be detected serologically. Paul et al. (1980) made serological studies on the relationship of some isometric virus of graminae. Discovery of antibodies 1899 *Jules Bordet, Complement and antibody activity in bacteriolysis 1900 *Paul Erlich, Antibody formation theory 1926 Lloyd Felton -
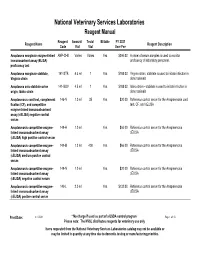
NVSL Reagent Manual
National Veterinary Services Laboratories Reagent Manual Reagent Amount/ Tests/ Billable FY 2021 Reagent Name Reagent Description Code Vial Vial User Fee Anaplasma marginale enzyme-linked ANP-CHK Varies VariesYes $394.00 A panel of serum samples is used to monitor immunosorbent assay (ELISA) proficiency of laboratory personnel. proficiency test Anaplasma marginale stabilate, 141-STB 4.5 ml 1Yes $188.00 Virginia strain, stabilate is used to initiate infection in Virginia strain donor animals Anaplasma ovis stabilate ovine 141-SOV 4.5 ml 1Yes $188.00 Idaho strain - stabilate is used to initate infection in origin, Idaho strain donor animals Anaplasmosis card test, complement 146-N 1.0 ml 35Yes $20.00 Reference control serum for the Anaplasmosis card fixation (CF), and competitive test, CF, and cELISA enzyme-linked immunoabsorbent assay (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-H 1.0 mlYes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) high positive control serum Anaplasmosis competitive enzyme- 149-M 1.0 ml 400Yes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) medium positve control serum Anaplasmosis competitive enzyme- 149-N 1.0 mlYes $20.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-L 2.0 mlYes $132.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) positve control serum Print Date: 6/11/2021 * No charge if used as part of a USDA control program Page 1 of 58 Please note: The NVSL distributes reagents for veterinary use only Items requested from the National Veterinary Services Laboratories catalog may not be available or may be limited in quantity at any time due to domestic testing or manufacturing priorities.