Interpretation of Serologic Tests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diagnostic Potential of the Haemagglutination
Bulgarian Journal of Veterinary Medicine (2007), 10 , N o 3, 169 −178 DIAGNOSTIC POTENTIAL OF THE HAEMAGGLUTINATION INHIBITION TEST, THE IMMUNODIFFUSION TEST AND ELISA FOR DETECTION OF ANTIBODIES IN CHICKENS, INTRAVENOUSLY INFECTED WITH A/DUCK/BULGARIA/05 H6N2 AVIAN INFLUENZA VIRUS ISOLATE I. S. ZARKOV Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria Summary Zarkov, I., 2007. Diagnostic potential of the haemagglutination inhibition test, the immuno- diffusion test and ELISA for detection of antibodies in chickens, intravenously infected with A/duck/Bulgaria/05 H6N2 avian influenza virus isolate. Bulg. J. Vet. Med. , 10 , No 3, 169 −178. After experimental infection of chickens with an avian influenza viral isolate A/duck/Bulgaria/05 H6N2, the potential of the haemagglutination inhibition test (HIT), the immunodiffusion test (IDT) and enzyme-linked immunosorbent assay (ELISA) for antibody detection was evaluated. The results evidenced that in birds, haemagglutinins, precipitins and IgG antibodies, detectable with ELISA, were formed. The percentage of chickens with subtype-specific antibodies was the highest by the 21 st day of infection (100 %), followed by the 14 th (66.7 %) and the 28 th (55.6 %) days, and was the lowest by the 7 th day (44.4 %). Serum titres ranged between 1:4 and 1:256 with predomination of 1:8 and 1:16 titres (29.2 % each). The mean arithmetic titre for the experiment was 1:38.2. The highest percentage of chickens with precipitins was observed by the 14th day (55.6 %) followed by the 7 th , 21 st and the 28 th days with 33.3 % each. -

Serological Methods in the Identification and Characterization of Viruses
CHAPTER 4 Serological Methods in the Identification and Characterization of Viruses M. H. V. Van Regenmortel Laboratoire de Virologie Institut de Biologie Mo!eculaire et Cellulaire 67000 Strasbourg, France 1. INTRODUCTION The purpose of this chapter is to present an integrated view of the various serological techniques that have been used in virology. The accent will be placed on the principles that govern each type of test and on the general applicability of the different serological techniques in all fields of virus research. In recent years, advances in serological tech niques have sometimes been applied in only one area of virology, although they could have been equally useful to workers studying other groups of viruses. No doubt this stems from the host-oriented approach that has guided the compartmentation of virology into separate fields of specialization. When it comes to serological properties, however, the similarities between animal, insect, bacterial, and plant viruses are paramount. The same immunochemical principles govern the in vitro serological reactions of all viral antigens, and much of general interest can be learned from the findings obtained with each particular group of viruses. An attempt will be made here to emphasize the general validity of specific experimental procedures. A number of recent reviews restricted to the serology of particular groups of viruses are available 183 H. Fraenkel-Conrat et al. (eds.), Comprehensive Virology © Plenum Press, New York 1981 184 Chapter 4 (Cowan, 1973; Schmidt and Lennette, 1973; Ball, 1974; Kurstak and Morisset, 1974; Burns and Allison, 1975; Mazzone and Tignor, 1976; Mayr et al., 1977; Tyrrell, 1978; Van Regenmortel, 1978; Cooper, 1979). -

Virology Techniques
Chapter 5 - Lesson 4 Virology Techniques Introduction Virology is a field within microbiology that encom- passes the study of viruses and the diseases they cause. In the laboratory, viruses have served as useful tools to better understand cellular mechanisms. The purpose of this lesson is to provide a general overview of laboratory techniques used in the identification and study of viruses. A Brief History In the late 19th century the independent work of Dimitri Ivanofsky and Martinus Beijerinck marked the begin- This electron micrograph depicts an influenza virus ning of the field of virology. They showed that the agent particle or virion. CDC. responsible for causing a serious disease in tobacco plants, tobacco mosaic virus, was able to pass through filters known to retain bacteria and the filtrate was able to cause disease in new plants. In 1898, Friedrich Loef- fler and Paul Frosch applied the filtration criteria to a disease in cattle known as foot and mouth disease. The filtration criteria remained the standard method used to classify an agent as a virus for nearly 40 years until chemical and physical studies revealed the structural basis of viruses. These attributes have become the ba- sis of many techniques used in the field today. Background All organisms are affected by viruses because viruses are capable of infecting and causing disease in all liv- ing species. Viruses affect plants, humans, and ani- mals as well as bacteria. A virus that infects bacteria is known as a bacteriophage and is considered the Bacteriophage. CDC. Chapter 5 - Human Health: Real Life Example (Influenza) 1 most abundant biological entity on the planet. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111 -
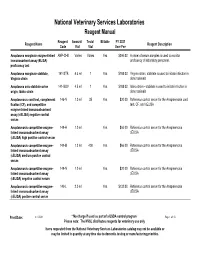
NVSL Reagent Manual
National Veterinary Services Laboratories Reagent Manual Reagent Amount/ Tests/ Billable FY 2021 Reagent Name Reagent Description Code Vial Vial User Fee Anaplasma marginale enzyme-linked ANP-CHK Varies VariesYes $394.00 A panel of serum samples is used to monitor immunosorbent assay (ELISA) proficiency of laboratory personnel. proficiency test Anaplasma marginale stabilate, 141-STB 4.5 ml 1Yes $188.00 Virginia strain, stabilate is used to initiate infection in Virginia strain donor animals Anaplasma ovis stabilate ovine 141-SOV 4.5 ml 1Yes $188.00 Idaho strain - stabilate is used to initate infection in origin, Idaho strain donor animals Anaplasmosis card test, complement 146-N 1.0 ml 35Yes $20.00 Reference control serum for the Anaplasmosis card fixation (CF), and competitive test, CF, and cELISA enzyme-linked immunoabsorbent assay (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-H 1.0 mlYes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) high positive control serum Anaplasmosis competitive enzyme- 149-M 1.0 ml 400Yes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) medium positve control serum Anaplasmosis competitive enzyme- 149-N 1.0 mlYes $20.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-L 2.0 mlYes $132.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) positve control serum Print Date: 6/11/2021 * No charge if used as part of a USDA control program Page 1 of 58 Please note: The NVSL distributes reagents for veterinary use only Items requested from the National Veterinary Services Laboratories catalog may not be available or may be limited in quantity at any time due to domestic testing or manufacturing priorities. -

A Hemagglutinin Quantification Method for Development of an Influenza Pandemic Vaccine Using Size Exclusion High Performance Liquid Chromatography
MOLECULAR MEDICINE REPORTS 11: 2819-2824, 2015 A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography HANG SIK ROH1, HYE MIN SONG1, BO REUM YUN1, HYUN KYUNG KANG1, KEUM SUK CHOI1, YUN JU PARK1, DONG SUB KIM1, SEUNG HEE KIM1, IN PIL MO2, BEUM‑SOO AN3 and CHI YOUNG AHN1 1Biologics Research Division, National Institute of Food and Drug Safety Evaluation, Korea Food and Drug Administration, Cheongwon‑gun, Chungcheongbuk‑do 363‑951; 2College of Veterinary Medicine, Chungbuk National University, Cheongju‑si, Chungcheongbuk‑do 361‑763; 3Department of Biomaterials Science, College of Natural Resources and Life Science, Pusan National University, Miryang, Gyeongsangnam‑do 627‑706, Republic of Korea Received February 17, 2014; Accepted November 19, 2014 DOI: 10.3892/mmr.2014.3049 Abstract. Single radial immunodiffusion (SRID) assay calculation based on SE‑HPLC exhibited 99.91‑100% simi- requires a reference antigen and an antibody to the hemag- larity, compared with that of SRID. These findings suggest glutinin (HA) of an influenza vaccine. As it takes 2‑3 months that the measurement of peak area ratio/HA content using to develop the reference antigen, vaccine development is SE‑HPLC may be a substitute for SRID and rapidly measure delayed in cases of an influenza pandemic. In the present HA content to enable faster development of a vaccine during study, the measurement of the HA content of influenza an influenza pandemic. vaccines was assessed using size exclusion high performance liquid chromatography (SE‑HPLC) for the rapid develop- Introduction ment of a pandemic vaccine. -

Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines
WHO/IVB/11.11 Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines Immunization, Vaccines and Biologicals WHO/IVB/11.11 Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines Immunization, Vaccines and Biologicals The Department of Immunization, Vaccines and Biologicals thanks the donors whose unspecified financial support has made the production of this document possible. This document was published by the Expanded Programme on Immunization (EPI) of the Department of Immunization, Vaccines and Biologicals Ordering code: WHO/IVB/11.11 Printed: April 2013 This publication is available on the Internet at: www.who.int/vaccines-documents/ Copies of this document as well as additional materials on immunization, vaccines and biologicals may be requested from: World Health Organization Department of Immunization, Vaccines and Biologicals CH-1211 Geneva 27, Switzerland • Fax: + 41 22 791 4227 • Email: [email protected] • © World Health Organization 2013 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Simple Serological Techniques"
LECTURE: 26 Title SIMPLE SEROLOGICAL LABORATORY TECHNIQUES LEARNING OBJECTIVES: The student should be able to: • Define the term "simple serological techniques". • Describe the benefit of the use of serological tests. • Define the term "titer". • Enumerate the environmental factors affecting the ag-ab interactions. • Enumerate the different immunological names give to antibodies. • Define the terms; prozone, equivalnce zone, and post zone. • Enumerate some examples of the major simple serological techniques, such as: - Agglutination reactions - Precipitation reactions • Explain the principle of the agglutination reactions • Enumerate some diagnostic test depend on the principle the of the agglutination reactions. • Explain the principle of the precipitation reactions • Enumerate some diagnostic test depend on the principle of the precipitation reaction. • Explain the terms; lattice, cross-reacting antibodies. Latex, charcoal, and agar. • Discuss the identity, partial identity, and non-identity • Compare between agglutination and precipitation reactions. • Explain the Immunodiffusion (single and double diffusion) methods (Ouchterlony technique). • Explain the principle of the toxin-anti-toxin reaction. • Explain the term "flocculation reaction". LECTURE REFRENCE: 1. TEXTBOOK: ROITT, BROSTOFF, MALE IMMUNOLOGY. 6th edition. Chapter 27. pg. 417-434. 2. TEXTBOOK: MARY LOUISE TURGEON. IMMUNOLOGY & SEROLOGY. IN LABORATORY MEDICINE. 2ND EDITION. Chapter 6. pg 111-131. 3. TEXTBOOK: ABUL K. ABBAS. ANDREW H. LICHTMAN. CELLULAR AND MOLECULAR IMMUNOLOGY. 5TH EDITION. pg 522-534. 1 SIMPLE LABORATORY METHODS I. GENERAL CONSIDERATIONS Serologic reactions that are in vitro Antigen-antibody reactions provide methods for the diagnosis of disease and for the identification and quantitation of antigens and antibodies. Simple serological techniques are called simple, because, these procedures involving direct demonstration and observation of reactions, they do not require the participation of accessory factors such as; indicator system, or specialized equipment. -

Immunology and Serology
LECTURE NOTES For Medical Laboratory Technology Students Immunology and Serology Selamawit Debebe Alemaya University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2004 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2004 by Selamawit Debebe All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Immunology and Serology Preface Immunology and serology is an advanced science dealing with how the human immune system organized, function and the different types of serological techniques. It is a very vast subject covering a wide area of technology. -

Latex Agglutination Tests for Selected Escherichia Coli Enzymes Mark John Wolcott Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1993 Latex agglutination tests for selected Escherichia coli enzymes Mark John Wolcott Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons, and the Microbiology Commons Recommended Citation Wolcott, Mark John, "Latex agglutination tests for selected Escherichia coli enzymes " (1993). Retrospective Theses and Dissertations. 10288. https://lib.dr.iastate.edu/rtd/10288 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

The Use of Transfer Factor in a Patient with Agammaglobulinemia
TRANSFER FACTOR IN AGAMMAGLOBULINEMIA 54 1 41. Murphy, B. E. P.: Some studies of the protein-binding of steroids and their (1972). application to the routine micro and ultramicro measurement of various 54. Tietze, H. U., Zurbriigg, R. P.. Zuppinger. K. A,, Joss. E. E.. and KZser, H.: steroids in body nuids by competitive protein-binding radioassay. J. Clin. Occurrence of impaired cortisol regulation in children with hypoglycemia Endocrinol. Metab.. 27: 973 (1967). associated with adrenal medullary hyporesponsiveness. J. Clin. Endocrinol. 42. Nelson, N.: A photometric adaptation of the Somogyi method for the determina- Metab., 34: 948 (1972). tion of glucose. J. Biol. Chem., 153: 375 (1944). 55. Unger, R. H.: High growth-hormone levels in diahet~cketoacidosis: A possible 43. Outschoorn. A. S.: The hormones of the adrenal medulla and their release. Brit. cause of insulin resistance. J. Amer. Med. Ass.. 191: 945 (1965). J. Pharmacol.. 7: 605 (1952). 56. Voorhess. M. L.: Urinary catecholamine excretion by healthy children. I. Daily 44. Perkoff, G. T., and Tyler, F. H.: Paradoxical hyperglycemia In diabetic patients excretion of dopamine, norepinephrine, epinephrine and 3-methoxy-4- treated with insulin. Metabolism, 3: 110 (1954). hydroxymandelic acid. Pediatrics, 39: 252 (1967). 45. Roth. J.. Glick, S. M., Yalow. R. S., and Berson, S. A,: Hypoglycemia: A potent 57. Wallace. J. M.. and Harlan. W. R.: Significance of epinephrine in insulin stimulus to secretion of growth hormone. Science, 140: 987 (1963). hypoglycemia in man. Amer. J. Med., 38: 531 (1965). 46. Sabeh, G., Mendelsohn, L. V.. Corredor, D. G.. Sunder, J. H., Friedman, L. M., 58. -

An Immuno-Assay to Quantify Influenza Virus Hemagglutinin with Correctly Folded Stalk Domains in Vaccine Preparations
RESEARCH ARTICLE An immuno-assay to quantify influenza virus hemagglutinin with correctly folded stalk domains in vaccine preparations Madhusudan Rajendran1,2, Weina Sun1, Phillip Comella1,2, Raffael Nachbagauer1, Teddy John Wohlbold1,2, Fatima Amanat1, Ericka Kirkpatrick1,2, Peter Palese1,3, Florian Krammer1* 1 Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States of America, 2 Graduate School of Biomedical Sciences, Icahn School of Medicine at Mount Sinai, New York, a1111111111 NY, United States of America, 3 Department of Medicine, Icahn School of Medicine at Mount Sinai, New a1111111111 York, NY, United States of America a1111111111 a1111111111 * [email protected] a1111111111 Abstract The standard method to quantify the hemagglutinin content of influenza virus vaccines is the OPEN ACCESS single radial immunodiffusion assay. This assay primarily relies on polyclonal antibodies Citation: Rajendran M, Sun W, Comella P, against the head domain of the influenza virus hemagglutinin, which is the main target anti- Nachbagauer R, Wohlbold TJ, Amanat F, et al. (2018) An immuno-assay to quantify influenza gen of influenza virus vaccines. virus hemagglutinin with correctly folded stalk Novel influenza virus vaccine candidates that redirect the immune response towards the domains in vaccine preparations. PLoS ONE 13(4): evolutionary more conserved hemagglutinin stalk, including chimeric hemagglutinin and e0194830. https://doi.org/10.1371/journal. headless hemagglutinin constructs, are highly dependent on the structural integrity of the pone.0194830 protein to present conformational epitopes for neutralizing antibodies. In this study, we Editor: Stefan PoÈhlmann, Deutsches describe a novel enzyme-linked immunosorbent assay that allows quantifying the amount of Primatenzentrum GmbH - Leibniz-Institut fur Primatenforschung, GERMANY hemagglutinin with correctly folded stalk domains and which could be further developed into a potency assay for stalk-based influenza virus vaccines.