Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Immune Effector Mechanisms and Designer Vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA
EXPERT REVIEW OF VACCINES https://doi.org/10.1080/14760584.2019.1674144 REVIEW How vaccines work: immune effector mechanisms and designer vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA ABSTRACT ARTICLE HISTORY Introduction: Three major advances have led to increase in length and quality of human life: Received 6 June 2019 increased food production, improved sanitation and induction of specific adaptive immune Accepted 25 September 2019 responses to infectious agents (vaccination). Which has had the most impact is subject to debate. KEYWORDS The number and variety of infections agents and the mechanisms that they have evolved to allow Vaccines; immune effector them to colonize humans remained mysterious and confusing until the last 50 years. Since then mechanisms; toxin science has developed complex and largely successful ways to immunize against many of these neutralization; receptor infections. blockade; anaphylactic Areas covered: Six specific immune defense mechanisms have been identified. neutralization, cytolytic, reactions; antibody- immune complex, anaphylactic, T-cytotoxicity, and delayed hypersensitivity. The role of each of these mediated cytolysis; immune immune effector mechanisms in immune responses induced by vaccination against specific infectious complex reactions; T-cell- mediated cytotoxicity; agents is the subject of this review. delayed hypersensitivity Expertopinion: In the past development of specific vaccines for infections agents was largely by trial and error. With an understanding of the natural history of an infection and the effective immune response to it, one can select the method of vaccination that will elicit the appropriate immune effector mechanisms (designer vaccines). These may act to prevent infection (prevention) or eliminate an established on ongoing infection (therapeutic). -

Diagnostic Potential of the Haemagglutination
Bulgarian Journal of Veterinary Medicine (2007), 10 , N o 3, 169 −178 DIAGNOSTIC POTENTIAL OF THE HAEMAGGLUTINATION INHIBITION TEST, THE IMMUNODIFFUSION TEST AND ELISA FOR DETECTION OF ANTIBODIES IN CHICKENS, INTRAVENOUSLY INFECTED WITH A/DUCK/BULGARIA/05 H6N2 AVIAN INFLUENZA VIRUS ISOLATE I. S. ZARKOV Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria Summary Zarkov, I., 2007. Diagnostic potential of the haemagglutination inhibition test, the immuno- diffusion test and ELISA for detection of antibodies in chickens, intravenously infected with A/duck/Bulgaria/05 H6N2 avian influenza virus isolate. Bulg. J. Vet. Med. , 10 , No 3, 169 −178. After experimental infection of chickens with an avian influenza viral isolate A/duck/Bulgaria/05 H6N2, the potential of the haemagglutination inhibition test (HIT), the immunodiffusion test (IDT) and enzyme-linked immunosorbent assay (ELISA) for antibody detection was evaluated. The results evidenced that in birds, haemagglutinins, precipitins and IgG antibodies, detectable with ELISA, were formed. The percentage of chickens with subtype-specific antibodies was the highest by the 21 st day of infection (100 %), followed by the 14 th (66.7 %) and the 28 th (55.6 %) days, and was the lowest by the 7 th day (44.4 %). Serum titres ranged between 1:4 and 1:256 with predomination of 1:8 and 1:16 titres (29.2 % each). The mean arithmetic titre for the experiment was 1:38.2. The highest percentage of chickens with precipitins was observed by the 14th day (55.6 %) followed by the 7 th , 21 st and the 28 th days with 33.3 % each. -

Medical Bacteriology
LECTURE NOTES Degree and Diploma Programs For Environmental Health Students Medical Bacteriology Abilo Tadesse, Meseret Alem University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education September 2006 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2006 by Abilo Tadesse, Meseret Alem All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE Text book on Medical Bacteriology for Medical Laboratory Technology students are not available as need, so this lecture note will alleviate the acute shortage of text books and reference materials on medical bacteriology. -

Serological Methods in the Identification and Characterization of Viruses
CHAPTER 4 Serological Methods in the Identification and Characterization of Viruses M. H. V. Van Regenmortel Laboratoire de Virologie Institut de Biologie Mo!eculaire et Cellulaire 67000 Strasbourg, France 1. INTRODUCTION The purpose of this chapter is to present an integrated view of the various serological techniques that have been used in virology. The accent will be placed on the principles that govern each type of test and on the general applicability of the different serological techniques in all fields of virus research. In recent years, advances in serological tech niques have sometimes been applied in only one area of virology, although they could have been equally useful to workers studying other groups of viruses. No doubt this stems from the host-oriented approach that has guided the compartmentation of virology into separate fields of specialization. When it comes to serological properties, however, the similarities between animal, insect, bacterial, and plant viruses are paramount. The same immunochemical principles govern the in vitro serological reactions of all viral antigens, and much of general interest can be learned from the findings obtained with each particular group of viruses. An attempt will be made here to emphasize the general validity of specific experimental procedures. A number of recent reviews restricted to the serology of particular groups of viruses are available 183 H. Fraenkel-Conrat et al. (eds.), Comprehensive Virology © Plenum Press, New York 1981 184 Chapter 4 (Cowan, 1973; Schmidt and Lennette, 1973; Ball, 1974; Kurstak and Morisset, 1974; Burns and Allison, 1975; Mazzone and Tignor, 1976; Mayr et al., 1977; Tyrrell, 1978; Van Regenmortel, 1978; Cooper, 1979). -

Module 2: Diphtheria
The Immunological Basis for Immunization Series Module 2: Diphtheria DEPARTMENT OF VACCINES AND BIOLOGICALS ~-) World Health Organization ~ ~ fJ! Geneva ----~ WHO/EPI/GEN/93.12 ORIGINAL: ENGLISH DISTR.: GENERAL The Immunological Basis for Immunization Series Module 2: Diphtheria Dr Artur M. Galazka Medical Officer Expanded Programme on Immunization DEPARTMENT OF VACCINES AND BIOLOGICALS .) World Health Organization ~ , ~ ~ Geneva ~ I iJff 2001 ~~ The Department of Vaccines and Biologicals thanks the donors whose unspecified financial support has made the production of this document possible. United Nations Development Fund (UNDP) The Rockefeller Foundation The Government of Sweden The Immunological Basis for Immunization series is available in English and French (from the address below). It has also been translated by national health authorities into a number of other languages for local use: Chinese, Italian, Persian, Russian, Turkish, Ukranian and Vietnamese. The series comprises eight independent modules: Module 1: General immunology Module 2: Diphtheria Module 3: Tetanus Module 4: Pertussis Module 5: Tuberculosis Module 6: Poliomyelitis Module 7: Measles Module 8: Yellow fever Produced in 1993 Reprinted (with new covers but no changes to content) in 2001 Ordering code: WHO/EPI/GEN/93.12 This document is available on the Internet at: www.who.int/vaccines-documents/ Copies may be requested from: World Health Organization Department of Vaccines and Biologicals CH-1211 Geneva 27, Switzerland • Fax: + 41 22 791 4227 • £-mail: [email protected] • ©World Health Organization 2001 This document is not a formal publication of the World Health Organization (WHO), and all rights are reserved by the Organization. The document may, however, be freely reviewed, abstracted, reproduced and translated, in part or in whole, but not for sale nor for use in conjunction with commercial purposes. -

Virology Techniques
Chapter 5 - Lesson 4 Virology Techniques Introduction Virology is a field within microbiology that encom- passes the study of viruses and the diseases they cause. In the laboratory, viruses have served as useful tools to better understand cellular mechanisms. The purpose of this lesson is to provide a general overview of laboratory techniques used in the identification and study of viruses. A Brief History In the late 19th century the independent work of Dimitri Ivanofsky and Martinus Beijerinck marked the begin- This electron micrograph depicts an influenza virus ning of the field of virology. They showed that the agent particle or virion. CDC. responsible for causing a serious disease in tobacco plants, tobacco mosaic virus, was able to pass through filters known to retain bacteria and the filtrate was able to cause disease in new plants. In 1898, Friedrich Loef- fler and Paul Frosch applied the filtration criteria to a disease in cattle known as foot and mouth disease. The filtration criteria remained the standard method used to classify an agent as a virus for nearly 40 years until chemical and physical studies revealed the structural basis of viruses. These attributes have become the ba- sis of many techniques used in the field today. Background All organisms are affected by viruses because viruses are capable of infecting and causing disease in all liv- ing species. Viruses affect plants, humans, and ani- mals as well as bacteria. A virus that infects bacteria is known as a bacteriophage and is considered the Bacteriophage. CDC. Chapter 5 - Human Health: Real Life Example (Influenza) 1 most abundant biological entity on the planet. -

Interpretation of Serologic Tests
COCCIDIOIDOMYCOSIS SEROLOGY LABORATORY School of Medicine, University of California, Davis, California, 95616-8645 George Thompson M.D., Director NPI: 1831206812 VETERINARY CLIENTS Application and Interpretation of Serologic Tests A. Our usual serologic tests for coccidioidomycosis are the following: 1. Qualitative: Immunodiffusion (ID) to determine presence of coccidioidal IgM ("precipitin") (test may be termed “IDTP”), or IgG (CF) (test may be termed “IDCF”) antibody in body fluids. This is appropriate if no diagnosis of coccidioidomycosis has previously been established. [Non-specific reactions resembling that given by the coccidioidal precipitin (IgM) are encountered infrequently with some sera, e.g. from patients who do not have coccidioidomycosis.] 2. Quantitative: For veterinary specimens, we estimate the titer of the CF (IgG) by quantitative immunodiffusion rather than by complement fixation (CF). This provides an approximation of the CF titer. The quantitative immunodiffusion rather than the CF test avoids the problem of anticomplementary activity frequently encountered especially with canine sera. Determination of titer is appropriate after a diagnosis has been established, e.g. by positive immunodiffusion or recovery of C. immitis from the patient. B. Results and interpretation: 1. Detection of coccidioidal antibody in a single specimen can be diagnostically significant; testing sequential samples can be helpful in following the course of the disease. 2. Immunodiffusion (ID) (qualitative) - presence or not of precipitin (IgM) or CF (IgG) antibody. The IgM is important in the diagnosis of acute primary coccidioidomycosis, and is detectable in most patients in 1 to 2 weeks after onset of symptoms, may persist several months, occasionally longer in association with a pulmonary cavity or disseminated disease. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111 -
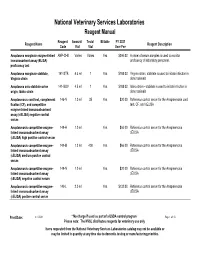
NVSL Reagent Manual
National Veterinary Services Laboratories Reagent Manual Reagent Amount/ Tests/ Billable FY 2021 Reagent Name Reagent Description Code Vial Vial User Fee Anaplasma marginale enzyme-linked ANP-CHK Varies VariesYes $394.00 A panel of serum samples is used to monitor immunosorbent assay (ELISA) proficiency of laboratory personnel. proficiency test Anaplasma marginale stabilate, 141-STB 4.5 ml 1Yes $188.00 Virginia strain, stabilate is used to initiate infection in Virginia strain donor animals Anaplasma ovis stabilate ovine 141-SOV 4.5 ml 1Yes $188.00 Idaho strain - stabilate is used to initate infection in origin, Idaho strain donor animals Anaplasmosis card test, complement 146-N 1.0 ml 35Yes $20.00 Reference control serum for the Anaplasmosis card fixation (CF), and competitive test, CF, and cELISA enzyme-linked immunoabsorbent assay (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-H 1.0 mlYes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) high positive control serum Anaplasmosis competitive enzyme- 149-M 1.0 ml 400Yes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) medium positve control serum Anaplasmosis competitive enzyme- 149-N 1.0 mlYes $20.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-L 2.0 mlYes $132.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) positve control serum Print Date: 6/11/2021 * No charge if used as part of a USDA control program Page 1 of 58 Please note: The NVSL distributes reagents for veterinary use only Items requested from the National Veterinary Services Laboratories catalog may not be available or may be limited in quantity at any time due to domestic testing or manufacturing priorities. -

A Hemagglutinin Quantification Method for Development of an Influenza Pandemic Vaccine Using Size Exclusion High Performance Liquid Chromatography
MOLECULAR MEDICINE REPORTS 11: 2819-2824, 2015 A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography HANG SIK ROH1, HYE MIN SONG1, BO REUM YUN1, HYUN KYUNG KANG1, KEUM SUK CHOI1, YUN JU PARK1, DONG SUB KIM1, SEUNG HEE KIM1, IN PIL MO2, BEUM‑SOO AN3 and CHI YOUNG AHN1 1Biologics Research Division, National Institute of Food and Drug Safety Evaluation, Korea Food and Drug Administration, Cheongwon‑gun, Chungcheongbuk‑do 363‑951; 2College of Veterinary Medicine, Chungbuk National University, Cheongju‑si, Chungcheongbuk‑do 361‑763; 3Department of Biomaterials Science, College of Natural Resources and Life Science, Pusan National University, Miryang, Gyeongsangnam‑do 627‑706, Republic of Korea Received February 17, 2014; Accepted November 19, 2014 DOI: 10.3892/mmr.2014.3049 Abstract. Single radial immunodiffusion (SRID) assay calculation based on SE‑HPLC exhibited 99.91‑100% simi- requires a reference antigen and an antibody to the hemag- larity, compared with that of SRID. These findings suggest glutinin (HA) of an influenza vaccine. As it takes 2‑3 months that the measurement of peak area ratio/HA content using to develop the reference antigen, vaccine development is SE‑HPLC may be a substitute for SRID and rapidly measure delayed in cases of an influenza pandemic. In the present HA content to enable faster development of a vaccine during study, the measurement of the HA content of influenza an influenza pandemic. vaccines was assessed using size exclusion high performance liquid chromatography (SE‑HPLC) for the rapid develop- Introduction ment of a pandemic vaccine. -

Simple Serological Techniques"
LECTURE: 26 Title SIMPLE SEROLOGICAL LABORATORY TECHNIQUES LEARNING OBJECTIVES: The student should be able to: • Define the term "simple serological techniques". • Describe the benefit of the use of serological tests. • Define the term "titer". • Enumerate the environmental factors affecting the ag-ab interactions. • Enumerate the different immunological names give to antibodies. • Define the terms; prozone, equivalnce zone, and post zone. • Enumerate some examples of the major simple serological techniques, such as: - Agglutination reactions - Precipitation reactions • Explain the principle of the agglutination reactions • Enumerate some diagnostic test depend on the principle the of the agglutination reactions. • Explain the principle of the precipitation reactions • Enumerate some diagnostic test depend on the principle of the precipitation reaction. • Explain the terms; lattice, cross-reacting antibodies. Latex, charcoal, and agar. • Discuss the identity, partial identity, and non-identity • Compare between agglutination and precipitation reactions. • Explain the Immunodiffusion (single and double diffusion) methods (Ouchterlony technique). • Explain the principle of the toxin-anti-toxin reaction. • Explain the term "flocculation reaction". LECTURE REFRENCE: 1. TEXTBOOK: ROITT, BROSTOFF, MALE IMMUNOLOGY. 6th edition. Chapter 27. pg. 417-434. 2. TEXTBOOK: MARY LOUISE TURGEON. IMMUNOLOGY & SEROLOGY. IN LABORATORY MEDICINE. 2ND EDITION. Chapter 6. pg 111-131. 3. TEXTBOOK: ABUL K. ABBAS. ANDREW H. LICHTMAN. CELLULAR AND MOLECULAR IMMUNOLOGY. 5TH EDITION. pg 522-534. 1 SIMPLE LABORATORY METHODS I. GENERAL CONSIDERATIONS Serologic reactions that are in vitro Antigen-antibody reactions provide methods for the diagnosis of disease and for the identification and quantitation of antigens and antibodies. Simple serological techniques are called simple, because, these procedures involving direct demonstration and observation of reactions, they do not require the participation of accessory factors such as; indicator system, or specialized equipment. -

Immunology and Serology
LECTURE NOTES For Medical Laboratory Technology Students Immunology and Serology Selamawit Debebe Alemaya University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2004 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2004 by Selamawit Debebe All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Immunology and Serology Preface Immunology and serology is an advanced science dealing with how the human immune system organized, function and the different types of serological techniques. It is a very vast subject covering a wide area of technology.