Kuby Immunology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
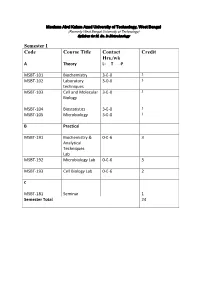
Syllabus for M
Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology Semester I Code Course Title Contact Credit Hrs./wk A Theory L- T -P MSBT-101 Biochemistry 3-0-0 3 MSBT-102 Laboratory 3-0-0 3 techniques MSBT-103 Cell and Molecular 3-0-0 3 Biology MSBT-104 Biostatistics 3-0-0 3 MSBT-105 Microbiology 3-0-0 3 B Practical MSBT-191 Biochemistry & 0-0-6 3 Analytical Techniques Lab MSBT-192 Microbiology Lab 0-0-6 3 MSBT-193 Cell Biology Lab 0-0-6 2 C MSBT-181 Seminar 1 Semester Total 24 Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology MSBT101: Biochemistry credits 3 Unit 1: Basic chemistry for biologists Formation of chemical bonds, molecular orbital (MO) theory and linear combination of atomic orbitals (LCAO),basics of mass spectrometry, molecules, Avogadro number, molarity, chemical reactions, reaction stoichiometry, rates of reaction, rate constants, order of reactions,kinetic versus thermodynamic controls of a reaction, reaction equilibrium (equilibrium constant); light and matter interactions (optical spectroscopy, fluorescence, bioluminescence, paramagnetism and diamagnetism, photoelectron spectroscopy; chemical bonds (ionic, covalent, Van derWalls forces); electronegativity, polarity; VSEPR theory and molecular geometry, dipole moment, orbital hybridizations; acids, bases and pH - Arrhenious theory, pH, ionic product of water, weak acids and bases, conjugate acid-base pairs, buffers and buffering action etc; chemical thermodynamics - internal energy, heat and temperature, enthalpy (bond enthalpy and reaction enthalpy), entropy, Gibbs free energy of ATP driven reactions, spontaneity versus driven reactions in biology;bond rotations and molecular conformations - Newman projections, conformational analysis of alkanes, alkenes and alkynes; functional groups, optically asymmetric carbon centers, amino acids, proteins, rotational freedoms in polypeptide backbone (Ramachandran plot). -

Technical Methods
J Clin Pathol 1987;40:581-588 J Clin Pathol: first published as 10.1136/jcp.40.5.581 on 1 May 1987. Downloaded from 56°C for 30 minutes. Technical methods Complement fixation tests were performed accord- ing to established methods,10 1 except that microtitre plates were used instead of World Health Organisation trays. For maximum sensitivity an ini- Cytomegalovirus (CMV) tial serum dilution of 1/4 was used. The antigen prep- antibody screening in blood aration used was a CMV complement fixation test antigen supplied by either Flow Laboratories Ltd, donors: modification of new latex Irvine, Scotland, or the Central Public Health Labo- ratory, Colindale, England. Guinea pig complements agglutination test compared with were supplied by Wellcome Diagnostics, Dartford, two standard methods England, or Don Whitly Scientific Ltd, Shipley, England. Complement fixation tests were performed A PUCKETT J E DAVIS From the Regional Blood using the following CMV antigen and complement Transfusion Centre, John Radeliffe Hospital, combinations: (1) PHLS CMV antigen + Wellcome Headington, Oxford, England Diagnostics complement, (2) PHLS CMV antigen + Don Whitly complement, and (3) Flow Laboratories CMV antigen + Wellcome Diagnostics complement. Infection with cytomegalovirus (CMV) is common, Immunofluorescence tests were performed and between 50 and 100% of adults may show evi- according to a standard method12 13 using substrate dence of infection.1 The transmission of the virus by slides of CMV infected (Westwood strain) fibroblasts blood transfusion2 and, therefore, the need to screen the Oxford Public Health Laboratory. donations intended for at risk groups such as provided by immunocompromised patients34 and neonates5 -7 iS CMV Scan passive latex agglutination kits were now well established. -

Diagnostic Potential of the Haemagglutination
Bulgarian Journal of Veterinary Medicine (2007), 10 , N o 3, 169 −178 DIAGNOSTIC POTENTIAL OF THE HAEMAGGLUTINATION INHIBITION TEST, THE IMMUNODIFFUSION TEST AND ELISA FOR DETECTION OF ANTIBODIES IN CHICKENS, INTRAVENOUSLY INFECTED WITH A/DUCK/BULGARIA/05 H6N2 AVIAN INFLUENZA VIRUS ISOLATE I. S. ZARKOV Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria Summary Zarkov, I., 2007. Diagnostic potential of the haemagglutination inhibition test, the immuno- diffusion test and ELISA for detection of antibodies in chickens, intravenously infected with A/duck/Bulgaria/05 H6N2 avian influenza virus isolate. Bulg. J. Vet. Med. , 10 , No 3, 169 −178. After experimental infection of chickens with an avian influenza viral isolate A/duck/Bulgaria/05 H6N2, the potential of the haemagglutination inhibition test (HIT), the immunodiffusion test (IDT) and enzyme-linked immunosorbent assay (ELISA) for antibody detection was evaluated. The results evidenced that in birds, haemagglutinins, precipitins and IgG antibodies, detectable with ELISA, were formed. The percentage of chickens with subtype-specific antibodies was the highest by the 21 st day of infection (100 %), followed by the 14 th (66.7 %) and the 28 th (55.6 %) days, and was the lowest by the 7 th day (44.4 %). Serum titres ranged between 1:4 and 1:256 with predomination of 1:8 and 1:16 titres (29.2 % each). The mean arithmetic titre for the experiment was 1:38.2. The highest percentage of chickens with precipitins was observed by the 14th day (55.6 %) followed by the 7 th , 21 st and the 28 th days with 33.3 % each. -

Serological Methods in the Identification and Characterization of Viruses
CHAPTER 4 Serological Methods in the Identification and Characterization of Viruses M. H. V. Van Regenmortel Laboratoire de Virologie Institut de Biologie Mo!eculaire et Cellulaire 67000 Strasbourg, France 1. INTRODUCTION The purpose of this chapter is to present an integrated view of the various serological techniques that have been used in virology. The accent will be placed on the principles that govern each type of test and on the general applicability of the different serological techniques in all fields of virus research. In recent years, advances in serological tech niques have sometimes been applied in only one area of virology, although they could have been equally useful to workers studying other groups of viruses. No doubt this stems from the host-oriented approach that has guided the compartmentation of virology into separate fields of specialization. When it comes to serological properties, however, the similarities between animal, insect, bacterial, and plant viruses are paramount. The same immunochemical principles govern the in vitro serological reactions of all viral antigens, and much of general interest can be learned from the findings obtained with each particular group of viruses. An attempt will be made here to emphasize the general validity of specific experimental procedures. A number of recent reviews restricted to the serology of particular groups of viruses are available 183 H. Fraenkel-Conrat et al. (eds.), Comprehensive Virology © Plenum Press, New York 1981 184 Chapter 4 (Cowan, 1973; Schmidt and Lennette, 1973; Ball, 1974; Kurstak and Morisset, 1974; Burns and Allison, 1975; Mazzone and Tignor, 1976; Mayr et al., 1977; Tyrrell, 1978; Van Regenmortel, 1978; Cooper, 1979). -

Msc Biochemistry Autonomy 2015-17
Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad–500094 Autonomous (Accredited with ‘A’ grade by NAAC) MSc Biochemistry Autonomy 2015-17 Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad–500094 Autonomous (Accredited with ‘A’ grade by NAAC) M.Sc. Biochemistry Syllabus (Effective from 2015 admitted batch) SEMESTER I PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I : BI101T:Chemistry and Metabolism of Proteins, Lipids and Porphyrins 4 4 30 70 2 Paper-II : BI102T:Chemistry and Metabolism of Carbohydrates, Vitamins 4 4 30 70 and Nucleic Acids 3 Paper-III: BI 103T: Bio-Analytical Techniques 4 4 30 70 4 Paper-IV: BI104T:BioenergeticsAndCellBiology 4 4 30 70 5 Paper-V: BI105P: Amino acids and protein analysis 8 4 -- 100 6 Paper-VI: BI106P: Carbohydrate and lipid analysis 8 4 -- 100 Total 32 24 120 480 SEMESTER II PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I:BI201T:Enzymology 4 4 30 70 2 Paper-II:BI202T:MolecularBiology 4 4 30 70 3 Paper-III:BI203T:BiochemicalGeneticsAndModelOrganisms 4 4 30 70 4 Paper-IV: B1 204T: Computational methods and Cell study methods 4 4 30 70 5 Paper-V:BI205P:Enzymology and Biochemical preparations 8 4 -- 100 6 Paper-VI:BI206P: Molecular Biology, Genetics and Quantitative Biology 8 4 -- 100 Total 32 24 120 480 SEMESTER III PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I:BI301T:GeneRegulation and Genetic Engineering 4 4 30 70 2 Paper-II:BI302T:Immunology and Immunotechnology 4 4 30 70 3 Paper-III:BI303T: Virology, Nutrition & Clinical Biochemistry. -

Virology Techniques
Chapter 5 - Lesson 4 Virology Techniques Introduction Virology is a field within microbiology that encom- passes the study of viruses and the diseases they cause. In the laboratory, viruses have served as useful tools to better understand cellular mechanisms. The purpose of this lesson is to provide a general overview of laboratory techniques used in the identification and study of viruses. A Brief History In the late 19th century the independent work of Dimitri Ivanofsky and Martinus Beijerinck marked the begin- This electron micrograph depicts an influenza virus ning of the field of virology. They showed that the agent particle or virion. CDC. responsible for causing a serious disease in tobacco plants, tobacco mosaic virus, was able to pass through filters known to retain bacteria and the filtrate was able to cause disease in new plants. In 1898, Friedrich Loef- fler and Paul Frosch applied the filtration criteria to a disease in cattle known as foot and mouth disease. The filtration criteria remained the standard method used to classify an agent as a virus for nearly 40 years until chemical and physical studies revealed the structural basis of viruses. These attributes have become the ba- sis of many techniques used in the field today. Background All organisms are affected by viruses because viruses are capable of infecting and causing disease in all liv- ing species. Viruses affect plants, humans, and ani- mals as well as bacteria. A virus that infects bacteria is known as a bacteriophage and is considered the Bacteriophage. CDC. Chapter 5 - Human Health: Real Life Example (Influenza) 1 most abundant biological entity on the planet. -

Interpretation of Serologic Tests
COCCIDIOIDOMYCOSIS SEROLOGY LABORATORY School of Medicine, University of California, Davis, California, 95616-8645 George Thompson M.D., Director NPI: 1831206812 VETERINARY CLIENTS Application and Interpretation of Serologic Tests A. Our usual serologic tests for coccidioidomycosis are the following: 1. Qualitative: Immunodiffusion (ID) to determine presence of coccidioidal IgM ("precipitin") (test may be termed “IDTP”), or IgG (CF) (test may be termed “IDCF”) antibody in body fluids. This is appropriate if no diagnosis of coccidioidomycosis has previously been established. [Non-specific reactions resembling that given by the coccidioidal precipitin (IgM) are encountered infrequently with some sera, e.g. from patients who do not have coccidioidomycosis.] 2. Quantitative: For veterinary specimens, we estimate the titer of the CF (IgG) by quantitative immunodiffusion rather than by complement fixation (CF). This provides an approximation of the CF titer. The quantitative immunodiffusion rather than the CF test avoids the problem of anticomplementary activity frequently encountered especially with canine sera. Determination of titer is appropriate after a diagnosis has been established, e.g. by positive immunodiffusion or recovery of C. immitis from the patient. B. Results and interpretation: 1. Detection of coccidioidal antibody in a single specimen can be diagnostically significant; testing sequential samples can be helpful in following the course of the disease. 2. Immunodiffusion (ID) (qualitative) - presence or not of precipitin (IgM) or CF (IgG) antibody. The IgM is important in the diagnosis of acute primary coccidioidomycosis, and is detectable in most patients in 1 to 2 weeks after onset of symptoms, may persist several months, occasionally longer in association with a pulmonary cavity or disseminated disease. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111 -

Comparison Between Haemagglutination Inhibition and Complement Fixation Tests in Detecting Antibodies Responses Following Influenza Viral Infection
Egypt. Acad. J. Biolog. Sci., 4(1): 35-38 (2012) G. Microbiology Email:[email protected] ISSN: 2090-0872 Received: 20/7/2012 www.eajbs.eg.net Comparison between Haemagglutination Inhibition and Complement Fixation Tests in Detecting Antibodies Responses Following Influenza Viral Infection Muthana, A.K. Al-zobaei Medical Microbiology, Microbiol. Dept. Virology, College of Medicine, Univ. Anbar. ABSTRACT Objectives: To evaluate sensitivity and specificity of HI and CF tests for detecting antibody responses following naturally occurring influenza A and B infections. Methods: One hundred and twenty blood samples were drawn from one hundred patients with flue-like illness and twenty apparently healthy control subjects and tested at Central Health Laboratory in Baghdad/ Iraq using HI and CF tests for detecting three currently circulating influenza A and B strains during the period from the 1st of January 2008 to the 1st of June 2009. Results: 78-80% of the patients with flu-like illness responded to a t least one of three influenza virus antigens as measured by HI, while 34-35% of those patients showed a response by CF test. Conclusions: HI test has a higher sensitivity and lower specificity than CF test for detecting antibody responses from patients with influenza A and B viral infections. Keywords : Influenza viruses, HI, CF, Influenza serology INTRODUCTION SD. (1986), whereas CF test based on Several studies have clearly fixation of complement by antigen- demonstrated that HI is a more sensitive antibody complexes. A known amount of method than CF test for detecting complement is included in the test and antibody responses to naturally occurring residual complement is detected by the influenza virus A and B infections, addition of sheep RBCs sensitized with Julkunen, I. -
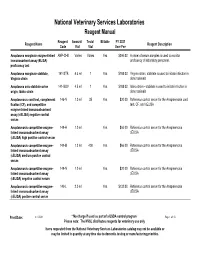
NVSL Reagent Manual
National Veterinary Services Laboratories Reagent Manual Reagent Amount/ Tests/ Billable FY 2021 Reagent Name Reagent Description Code Vial Vial User Fee Anaplasma marginale enzyme-linked ANP-CHK Varies VariesYes $394.00 A panel of serum samples is used to monitor immunosorbent assay (ELISA) proficiency of laboratory personnel. proficiency test Anaplasma marginale stabilate, 141-STB 4.5 ml 1Yes $188.00 Virginia strain, stabilate is used to initiate infection in Virginia strain donor animals Anaplasma ovis stabilate ovine 141-SOV 4.5 ml 1Yes $188.00 Idaho strain - stabilate is used to initate infection in origin, Idaho strain donor animals Anaplasmosis card test, complement 146-N 1.0 ml 35Yes $20.00 Reference control serum for the Anaplasmosis card fixation (CF), and competitive test, CF, and cELISA enzyme-linked immunoabsorbent assay (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-H 1.0 mlYes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) high positive control serum Anaplasmosis competitive enzyme- 149-M 1.0 ml 400Yes $66.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) medium positve control serum Anaplasmosis competitive enzyme- 149-N 1.0 mlYes $20.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) negative control serum Anaplasmosis competitive enzyme- 149-L 2.0 mlYes $132.00 Reference control serum for the Anaplasmosis linked immunoabsorbent assay cELISA (cELISA) positve control serum Print Date: 6/11/2021 * No charge if used as part of a USDA control program Page 1 of 58 Please note: The NVSL distributes reagents for veterinary use only Items requested from the National Veterinary Services Laboratories catalog may not be available or may be limited in quantity at any time due to domestic testing or manufacturing priorities. -

Clinical Reactivity and Immunogenicity of Hemagglutinin Influenza Vaccine
Archiv fiir die gesamte Virusforschung 36, 43--52 (1972) by Springer-Verlag 1972 Clinical Reactivity and Immunogenicity of Hemagglutinin Influenza Vaccine I. Clinical Reactions, Hemagghtination-Inhibiting and Strain and Type-Specific Complement-Fixing Antibody Responses in Subjects Aged 3--6, 16--17 and 27--50 Years By HANA ZJ~VADOVX, VLADIM~I/r VONKA, EnvI~ ADAM, EvA DOMOR•ZKOV•, and FRED M. DAVENPORT1 Department of Experimental Virology and Clinical and Epidemiology Department, Institute of Sera and Vaccines, Prague, Czechoslovakia Received April 5, 1971 Summary Subjects aged 3--6, 16--17 and 27--50 years were vaccinated with one dose of hemagglutinin influenza virus vaccine. Clinical reactions, hemagglutination- inhibiting (HI) and strain- and type-specific complement-fixing (CF-V and CF-S) antibodies were determined in sera taken before and four weeks after vaccine administration. The results indicated that the reactogenicity of the vaccine was very low. The HI antibody response differed With the age of the vaceinees, apparently being conditioned by prior exposure to the various influenza virus subtypes. The results of CF tests using strain-specific V antigens corresponded in general with HI tests, with two marked exceptions. In the youngest group nearly half of the subjects developed CF antibody to V-Swine, while all of them remained without antibody detectable in the HI test. However, when V antigen was used instead of intact virus as hemagglutinin, the post-vaccination sera of these subjects also reacted positively in the HI test. Secondly, a number of pre- vaccination sera from persons aged 27--50 years possessed CF antibody to A/PR 8 in the: absence of homologous HI antibody. -

A Hemagglutinin Quantification Method for Development of an Influenza Pandemic Vaccine Using Size Exclusion High Performance Liquid Chromatography
MOLECULAR MEDICINE REPORTS 11: 2819-2824, 2015 A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography HANG SIK ROH1, HYE MIN SONG1, BO REUM YUN1, HYUN KYUNG KANG1, KEUM SUK CHOI1, YUN JU PARK1, DONG SUB KIM1, SEUNG HEE KIM1, IN PIL MO2, BEUM‑SOO AN3 and CHI YOUNG AHN1 1Biologics Research Division, National Institute of Food and Drug Safety Evaluation, Korea Food and Drug Administration, Cheongwon‑gun, Chungcheongbuk‑do 363‑951; 2College of Veterinary Medicine, Chungbuk National University, Cheongju‑si, Chungcheongbuk‑do 361‑763; 3Department of Biomaterials Science, College of Natural Resources and Life Science, Pusan National University, Miryang, Gyeongsangnam‑do 627‑706, Republic of Korea Received February 17, 2014; Accepted November 19, 2014 DOI: 10.3892/mmr.2014.3049 Abstract. Single radial immunodiffusion (SRID) assay calculation based on SE‑HPLC exhibited 99.91‑100% simi- requires a reference antigen and an antibody to the hemag- larity, compared with that of SRID. These findings suggest glutinin (HA) of an influenza vaccine. As it takes 2‑3 months that the measurement of peak area ratio/HA content using to develop the reference antigen, vaccine development is SE‑HPLC may be a substitute for SRID and rapidly measure delayed in cases of an influenza pandemic. In the present HA content to enable faster development of a vaccine during study, the measurement of the HA content of influenza an influenza pandemic. vaccines was assessed using size exclusion high performance liquid chromatography (SE‑HPLC) for the rapid develop- Introduction ment of a pandemic vaccine.