Epidemics and Pandemics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Poxvirus and Rabies Branch (PRB) Fact Sheet
Poxvirus & Rabies Branch (PRB) The epidemiologists, laboratory scientists, and public health professionals in PRB are responsible for surveillance, control, and prevention of illness and death due to poxviruses and rabies. Our Work Laboratory Reference, Diagnostics, & Training PRB’s provides laboratory reference, diagnostic services, and trainings to state and local health departments, the Department of Defense (DOD), international organizations, and the Laboratory Response Network (over 150 labs across the U.S. prepared to respond to bioterrorism). PRB collaborates with the private sector to develop and evaluate novel diagnostic assays, therapeutics, and vaccines for rabies and pox-related viruses. The branch maintains high-containment laboratories (BSL3 &4) to safely conduct public health research. Research & Surveillance PRB conducts research studies on the microbiology, molecular biology, pathogenesis (disease process), ecology, and evolution of Our Pathogens poxviruses and rabies. The branch monitors the incidence rates of PRB’s Poxvirus Team provides subject matter these diseases in the U.S. and internationally. expertise on: Expert Consultation Orthopoxviruses, including PRB provides consultation regarding poxvirus and rabies- Smallpox associated diseases and their diagnoses, prevention, control, and Monkeypox treatments. Assistance is offered to healthcare providers, academic Cowpox institutions, state and local health departments, other government Vaccinia Virus agencies, and the general public. PRB collaborates with non- governmental organizations to develop risk communication Parapoxviruses, including tools for disease prevention. The branch provides training on Orf Virus simple surveillance techniques and trains healthcare workers on monkeypox in the Democratic Republic of the Congo (DRC). PRB Pseudocowpox collaborates with the national government of Haiti on a rabies surveillance and control program which includes health education, enhanced diagnostics, surveillance, and an extensive dog vaccination campaign. -

Chikungunya Fever: Epidemiology, Clinical Syndrome, Pathogenesis
Antiviral Research 99 (2013) 345–370 Contents lists available at SciVerse ScienceDirect Antiviral Research journal homepage: www.elsevier.com/locate/antiviral Review Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy ⇑ Simon-Djamel Thiberville a,b, , Nanikaly Moyen a,b, Laurence Dupuis-Maguiraga c,d, Antoine Nougairede a,b, Ernest A. Gould a,b, Pierre Roques c,d, Xavier de Lamballerie a,b a UMR_D 190 ‘‘Emergence des Pathologies Virales’’ (Aix-Marseille Univ. IRD French Institute of Research for Development EHESP French School of Public Health), Marseille, France b University Hospital Institute for Infectious Disease and Tropical Medicine, Marseille, France c CEA, Division of Immuno-Virologie, Institute of Emerging Diseases and Innovative Therapies, Fontenay-aux-Roses, France d UMR E1, University Paris Sud 11, Orsay, France article info abstract Article history: Chikungunya virus (CHIKV) is the aetiological agent of the mosquito-borne disease chikungunya fever, a Received 7 April 2013 debilitating arthritic disease that, during the past 7 years, has caused immeasurable morbidity and some Revised 21 May 2013 mortality in humans, including newborn babies, following its emergence and dispersal out of Africa to the Accepted 18 June 2013 Indian Ocean islands and Asia. Since the first reports of its existence in Africa in the 1950s, more than Available online 28 June 2013 1500 scientific publications on the different aspects of the disease and its causative agent have been pro- duced. Analysis of these publications shows that, following a number of studies in the 1960s and 1970s, Keywords: and in the absence of autochthonous cases in developed countries, the interest of the scientific commu- Chikungunya virus nity remained low. -

Antibody-Mediated Enhancement Aggravates Chikungunya Virus
www.nature.com/scientificreports OPEN Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity Received: 14 July 2017 Fok-Moon Lum 1,2, Thérèse Couderc3,4, Bing-Shao Chia1,8, Ruo-Yan Ong1,9, Zhisheng Her1,10, Accepted: 17 January 2018 Angela Chow5, Yee-Sin Leo5, Yiu-Wing Kam1, Laurent Rénia1, Marc Lecuit 3,4,6 & Published: xx xx xxxx Lisa F. P. Ng1,2,7 The arthropod-transmitted chikungunya virus (CHIKV) causes a fu-like disease that is characterized by incapacitating arthralgia. The re-emergence of CHIKV and the continual risk of new epidemics have reignited research in CHIKV pathogenesis. Virus-specifc antibodies have been shown to control virus clearance, but antibodies present at sub-neutralizing concentrations can also augment virus infection that exacerbates disease severity. To explore this occurrence, CHIKV infection was investigated in the presence of CHIKV-specifc antibodies in both primary human cells and a murine macrophage cell line, RAW264.7. Enhanced attachment of CHIKV to the primary human monocytes and B cells was observed while increased viral replication was detected in RAW264.7 cells. Blocking of specifc Fc receptors (FcγRs) led to the abrogation of these observations. Furthermore, experimental infection in adult mice showed that animals had higher viral RNA loads and endured more severe joint infammation in the presence of sub-neutralizing concentrations of CHIKV-specifc antibodies. In addition, CHIKV infection in 11 days old mice under enhancing condition resulted in higher muscles viral RNA load detected and death. These observations provide the frst evidence of antibody-mediated enhancement in CHIKV infection and pathogenesis and could also be relevant for other important arboviruses such as Zika virus. -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -

Imported Monkeypox, Singapore
DISPATCHES Imported Monkeypox, Singapore Sarah Ee Fang Yong, Oon Tek Ng, Zheng Jie Marc Ho, Tze Minn Mak, Kalisvar Marimuthu, Shawn Vasoo, Tsin Wen Yeo, Yi Kai Ng, Lin Cui, Zannatul Ferdous, Po Ying Chia, Bryan Jun Wei Aw, Charmaine Malenab Manauis, Constance Khia Ki Low, Guanhao Chan, Xinyi Peh, Poh Lian Lim, Li Ping Angela Chow, Monica Chan, Vernon Jian Ming Lee, Raymond Tzer Pin Lin, Mok Kwee Derrick Heng, Yee Sin Leo In May 2019, we investigated monkeypox in a traveler and public health management for this case, together from Nigeria to Singapore. The public health response with lessons learned and implications for control. included rapid identification of contacts, use of quaran- tine, and postexposure smallpox vaccination. No sec- The Case ondary cases were identified. Countries should develop On May 8, 2019, monkeypox was laboratory-con- surveillance systems to detect emerging infectious dis- firmed in a 38-year-old man from Nigeria who had eases globally. traveled to Singapore. The man resided in Delta State, Nigeria, but had attended a wedding in Ebonyi State onkeypox is a zoonosis endemic to West and during April 21–23, where he reported ingestion of MCentral Africa; human cases were first report- barbecued bushmeat that might have been contami- ed in 1970 (1). An outbreak ongoing in Nigeria since nated. He did not handle raw meat and had no expo- 2017 is the largest documented (2). Exported cases in sure to wild animals or their products. He held an ad- the United Kingdom and Israel were reported from ministrative job and reported no contact with rodents travelers infected in Nigeria in 2018 (3,4). -

Clinical Scholars Visit Amish Research Clinic in Lancaster County, PA by Patrick Brunner, MD Dr
Spring 2018 Center for Clinical and Translational Science e- e-NewsletterNewsletter Center News James Krueger and Marina Caskey, Representing the Nussenzweig Lab Team, Honored at Translational Science 2018 Meeting By Hospital Leadership which fulfill the translation research Dr. James Krueger received paradigm of going from bench to the Association for Clinical and bedside, have been recognized by this Translational Science (ACTS) most prestigious national recognition. Distinguished Investigator Award for his It is well deserved!” Upon receiving the groundbreaking research on psoriasis at award, Dr. Krueger noted, “This award the Translational Science 2018 meeting would not have been possible without attended by more than 1,100 people in the support of many others associated Washington, DC in April. His research with the Rockefeller CTSA enterprise: has led to a fundamental change in my lab members, the nursing staff, the paradigm for understanding the all other support departments and, pathophysiology of the disorder, and of course, hundreds of patients who this in turn has led to the development directly tested progressively better drugs of a series of novel medications that that are now used to so effectively treat precisely modulate the immune system psoriasis.” and dramatically improve the therapy of the disorder. Each year, Clinical Research Forum sponsors a competition to identify Dr. Caskey Receiving “Top Ten” Award from the “Top Ten” Clinical Research Drs. Harry Selker and Herb Pardes studies reported in the previous year. commented that “The study reflects the The competition is intense and so it very best in translational science: the is a true tribute to the novelty and careful analysis of patient phenotypes; importance of the study led by Dr. -

Prevent Dengue & Chikungunya
Prevent Dengue & Chikungunya WHO Myanmar newsletter special, 9 September 2019 What is dengue? Dengue situation in Myanmar Dengue is one of the most common mosquito- Cases and deaths 2015-2019 borne viral infections. The infection is spread from one person to another through the bite of infectious mosquitoes, either Aedes aegypti or Aedes albopictus. There are 4 distinct types of dengue virus, called DEN-1, DEN-2, DEN-3 and DEN-4. Dengue can be mild, as in most cases, or severe, as in few cases. It can cause serious illness and death among children. In Myanmar, July to August is peak season for transmission of dengue - coinciding with monsoon. How every one of us can help prevent dengue source: VBDC, Department of Public Health, Ministry of Health & Sports, 2019 z community awareness for mosquito breeding As shown above, dengue is cyclical in Myanmar, control is key alternating annually, as is the case in many countries. z cover, empty and clean domestic water storage At the same time, the number of dengue cases, and containers -- weekly is best deaths due to denge, over the period 2015 to 2018, z remove from the environment plastic bottles, both show a decreasing trend overall. plastic bags, fruit cans, discarded tyres -- for these are preferred mosquito breeding sites by Examples of how national and local authorities aedes aegypti (depicted) and aedes albopictus contribute z drain water collection points around the house z providing support for dengue management at z seek advice of a health professional if you have health facilities. multiple signs or symptoms of dengue z strengthening online reporting of dengue, How to recognize dengue? what are signs and using electronic based information system. -
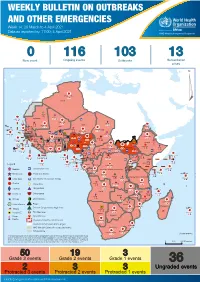
Weekly Bulletin on Outbreaks
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 14: 29 March to 4 April 2021 Data as reported by: 17:00; 4 April 2021 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 0 116 103 13 New event Ongoing events Outbreaks Humanitarian crises 117 622 3 105 Algeria ¤ 36 13 110 0 5 420 164 Mauritania 7 2 10 501 392 110 0 7 0 Niger 17 927 449 Mali 3 334 10 567 0 6 0 2 079 4 4 595 165 Eritrea Cape Verde 38 520 1 037 Chad Senegal 4 918 185 59 0 Gambia 27 0 3 0 17 125 159 9 761 45 Guinea-Bissau 796 17 7 0 Burkina Faso 225 46 215 189 2 963 0 162 593 2 048 Guinea 12 817 150 12 38 397 1 3 662 66 1 1 23 12 Benin 30 0 Nigeria 1 873 71 0 Ethiopia 420 14 481 5 6 188 15 Sierra Leone Togo 3 473 296 53 920 779 52 14 Ghana 5 245 72 Côte d'Ivoire 10 098 108 14 484 479 63 0 40 0 Liberia 17 0 South Sudan Central African Republic 916 2 45 0 25 0 19 670 120 43 180 237 90 287 740 Cameroon 7 0 28 676 137 5 330 13 138 988 2 224 1 952 87 655 2 51 22 43 0 112 12 6 1 488 6 3 988 79 11 187 6 902 102 Equatorial Guinea Uganda 542 8 Sao Tome and Principe 32 11 2 042 85 41 016 335 Kenya Legend 7 100 90 Gabon Congo 18 504 301 Rwanda Humanitarian crisis 2 212 34 22 482 311 Measles 18 777 111 Democratic Republic of the Congo 9 681 135 Burundi 2 964 6 Monkeypox Ebola virus disease Seychelles 27 930 739 1 525 0 420 29 United Republic of Tanzania Lassa fever Skin disease of unknown etiology 189 0 4 084 20 509 21 Cholera Yellow fever 1 349 5 6 257 229 22 631 542 cVDPV2 Dengue fever 88 930 1 220 Comoros Angola Malawi COVID-19 Chikungunya 33 661 1 123 862 0 3 719 146 -

Report on Global Surveillance of Epidemic-Prone Infectious Diseases, 2000
Pan American Health Organization Regional Office of the World Health Organization PAHO/DPC/CD-V/243/03 Original: English Report: Workshop on Dengue Burden Studies (Washington, DC, 5-7 November 2002) Convened by The Pan American Health Organization The Rockefeller Foundation The Pediatric Dengue Vaccine Initiative Executive Summary Background Dengue fever (DF) and dengue hemorrhagic fever (DHF) are caused by the mosquito borne virus, dengue virus, of which there are four antigenically distinct serotypes. It is estimated that annually these viruses cause at least 20 million infections worldwide leading to some 24,000 deaths (WHO, http://www.who.int/health_topics/dengue/en/ ). The alarming rise in dengue hemorrhagic fever in the world today is illustrated most starkly by the chart below which represents data from the World Health Organization (WHO) showing the rise of DHF cases over the last four decades. Indeed the first two years of the new millennium has seen outbreak after outbreak of DHF not only in Southeast Asia where DHF has been seen for half a century, but also in many countries of South and Central America. REPORTED CASES OF DHF 600 ds 500 housan t 400 n d i e 300 200 r report e 100 numb 0 1955-1959 1960-1069 1970-1979 1980-1989 1990-1998 Source: WHO; adapted from http://www.who.int/health_topics/dengue/en/ While there is no doubt that severe dengue is spreading from countries in Southeast Asia to countries in the Pacific and in the Americas, there is also no doubt that many international efforts into the development of dengue vaccines have led to a number of promising vaccine candidates which may offer some solutions to the control of this disease. -

Monkeypox Virus Emergence in Wild Chimpanzees Reveals Distinct Clinical Outcomes and Viral Diversity
ARTICLES https://doi.org/10.1038/s41564-020-0706-0 Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity Livia V. Patrono1,6, Kamilla Pléh1,2,6, Liran Samuni2,3, Markus Ulrich1, Caroline Röthemeier1, Andreas Sachse1, Silvia Muschter4, Andreas Nitsche4, Emmanuel Couacy-Hymann5, Christophe Boesch2,3, Roman M. Wittig 2,3, Sébastien Calvignac-Spencer1 and Fabian H. Leendertz 1 ✉ Monkeypox is a viral zoonotic disease on the rise across endemic habitats. Despite the growing importance of monkeypox virus, our knowledge on its host spectrum and sylvatic maintenance is limited. Here, we describe the recent repeated emergence of monkeypox virus in a wild, human-habituated western chimpanzee (Pan troglodytes verus, hereafter chimpanzee) population from Taï National Park, Ivory Coast. Through daily monitoring, we show that further to causing its typical exanthematous syn- drome, monkeypox can present itself as a severe respiratory disease without a diffuse rash. By analysing 949 non-invasively collected samples, we identify the circulation of at least two distinct monkeypox virus lineages and document the shedding of infectious particles in faeces and flies, suggesting that they could mediate indirect transmission. We also show that the carnivo- rous component of the Taï chimpanzees’ diet, mainly consisting of the sympatric monkeys they regularly hunt, did not change nor shift towards rodent consumption (the presumed reservoir) before the outbreaks, suggesting that the sudden emergence of monkeypox virus in this population is probably due to changes in the ecology of the virus itself. Using long-term mortality surveillance data from Taï National Park, we provide evidence of little to no prior viral activity over at least two decades.