Antibody-Mediated Enhancement Aggravates Chikungunya Virus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Chikungunya Fever: Epidemiology, Clinical Syndrome, Pathogenesis
Antiviral Research 99 (2013) 345–370 Contents lists available at SciVerse ScienceDirect Antiviral Research journal homepage: www.elsevier.com/locate/antiviral Review Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy ⇑ Simon-Djamel Thiberville a,b, , Nanikaly Moyen a,b, Laurence Dupuis-Maguiraga c,d, Antoine Nougairede a,b, Ernest A. Gould a,b, Pierre Roques c,d, Xavier de Lamballerie a,b a UMR_D 190 ‘‘Emergence des Pathologies Virales’’ (Aix-Marseille Univ. IRD French Institute of Research for Development EHESP French School of Public Health), Marseille, France b University Hospital Institute for Infectious Disease and Tropical Medicine, Marseille, France c CEA, Division of Immuno-Virologie, Institute of Emerging Diseases and Innovative Therapies, Fontenay-aux-Roses, France d UMR E1, University Paris Sud 11, Orsay, France article info abstract Article history: Chikungunya virus (CHIKV) is the aetiological agent of the mosquito-borne disease chikungunya fever, a Received 7 April 2013 debilitating arthritic disease that, during the past 7 years, has caused immeasurable morbidity and some Revised 21 May 2013 mortality in humans, including newborn babies, following its emergence and dispersal out of Africa to the Accepted 18 June 2013 Indian Ocean islands and Asia. Since the first reports of its existence in Africa in the 1950s, more than Available online 28 June 2013 1500 scientific publications on the different aspects of the disease and its causative agent have been pro- duced. Analysis of these publications shows that, following a number of studies in the 1960s and 1970s, Keywords: and in the absence of autochthonous cases in developed countries, the interest of the scientific commu- Chikungunya virus nity remained low. -

NSP4)-Induced Intrinsic Apoptosis
viruses Article Viperin, an IFN-Stimulated Protein, Delays Rotavirus Release by Inhibiting Non-Structural Protein 4 (NSP4)-Induced Intrinsic Apoptosis Rakesh Sarkar †, Satabdi Nandi †, Mahadeb Lo, Animesh Gope and Mamta Chawla-Sarkar * Division of Virology, National Institute of Cholera and Enteric Diseases, P-33, C.I.T. Road Scheme-XM, Beliaghata, Kolkata 700010, India; [email protected] (R.S.); [email protected] (S.N.); [email protected] (M.L.); [email protected] (A.G.) * Correspondence: [email protected]; Tel.: +91-33-2353-7470; Fax: +91-33-2370-5066 † These authors contributed equally to this work. Abstract: Viral infections lead to expeditious activation of the host’s innate immune responses, most importantly the interferon (IFN) response, which manifests a network of interferon-stimulated genes (ISGs) that constrain escalating virus replication by fashioning an ill-disposed environment. Interestingly, most viruses, including rotavirus, have evolved numerous strategies to evade or subvert host immune responses to establish successful infection. Several studies have documented the induction of ISGs during rotavirus infection. In this study, we evaluated the induction and antiviral potential of viperin, an ISG, during rotavirus infection. We observed that rotavirus infection, in a stain independent manner, resulted in progressive upregulation of viperin at increasing time points post-infection. Knockdown of viperin had no significant consequence on the production of total Citation: Sarkar, R.; Nandi, S.; Lo, infectious virus particles. Interestingly, substantial escalation in progeny virus release was observed M.; Gope, A.; Chawla-Sarkar, M. upon viperin knockdown, suggesting the antagonistic role of viperin in rotavirus release. Subsequent Viperin, an IFN-Stimulated Protein, studies unveiled that RV-NSP4 triggered relocalization of viperin from the ER, the normal residence Delays Rotavirus Release by Inhibiting of viperin, to mitochondria during infection. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -

Identification and Characterisation of Human Cytomegalovirus-Mediated Degradation of Helicase-Like Transcription Factor
Identification and Characterisation of Human Cytomegalovirus-Mediated Degradation of Helicase-Like Transcription Factor Kai-Min Lin Department of Medicine Cambridge Institute for Medical Research University of Cambridge This dissertation is submitted for the degree of Doctor of Philosophy Fitzwilliam College September 2020 Declaration I hereby declare, that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree of qualification in this, or any other university. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration except as where specified in the text and acknowledgments. This dissertation does not exceed the specified word limit of 60,000 words as defined by the Degree Committee, excluding figures, photographs, tables, appendices and bibliography. Kai-Min Lin September, 2020 I Summary Identification and characterisation of human cytomegalovirus-mediated degradation of helicase-like transcription factor Kai-Min Lin Viruses are known to degrade host factors that are important in innate antiviral immunity in order to infect successfully. To systematically identify host proteins targeted for early degradation by human cytomegalovirus (HCMV), the lab developed orthogonal screens using high resolution multiplexed mass spectrometry. Taking advantage of broad and selective proteasome and lysosome inhibitors, proteasomal degradation was found to be heavily exploited by HCMV. Several known antiviral restriction factors, including components of cellular promyelocytic leukemia (PML) were enriched in a shortlist of proteasomally degraded proteins during infection. A particularly robust novel ‘hit’ was helicase-like transcription factor (HLTF), a DNA repair protein that participates in error-free repair of stalled replication forks. -

Antivirals Against the Chikungunya Virus
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 10 June 2021 Review Antivirals against the Chikungunya Virus Verena Battisti 1, Ernst Urban 2 and Thierry Langer 3,* 1 University of Vienna, Department of Pharmaceutical Sciences, Pharmaceutical Chemistry Division, A-1090 Vienna, Austria; [email protected] 2 University of Vienna, Department of Pharmaceutical Sciences, Pharmaceutical Chemistry Division, A-1090 Vienna, Austria; [email protected] 3 University of Vienna, Department of Pharmaceutical Sciences, Pharmaceutical Chemistry Division, A-1090 Vienna, Austria; * Correspondence: [email protected] Abstract: Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that has re-emerged in recent decades, causing large-scale epidemics in many parts of the world. CHIKV infection leads to a febrile disease known as chikungunya fever (CHIKF), which is characterised by severe joint pain and myalgia. As many patients develop a painful chronic stage and neither antiviral drugs nor vac- cines are available, the development of a potent CHIKV inhibiting drug is crucial for CHIKF treat- ment. A comprehensive summary of current antiviral research and development of small-molecule inhibitor against CHIKV is presented in this review. We highlight different approaches used for the identification of such compounds and further discuss the identification and application of promis- ing viral and host targets. Keywords: Chikungunya virus ; alphavirus; antiviral therapy; direct-acting antivirals; host-directed antivirals; in silico screening; in vivo validation, antiviral drug development 1. Introduction Chikungunya virus (CHIKV) is a mosquito-borne alphavirus and belongs to the Togaviridae family. The virus was first isolated from a febrile patient in 1952/53 in the Makonde plateau (Tanzania) and has been named after the Makonde word for “that which bends you up”, describing the characteristic posture of patients suffering severe joint pains due to the CHIKV infection [1]. -

Prevent Dengue & Chikungunya
Prevent Dengue & Chikungunya WHO Myanmar newsletter special, 9 September 2019 What is dengue? Dengue situation in Myanmar Dengue is one of the most common mosquito- Cases and deaths 2015-2019 borne viral infections. The infection is spread from one person to another through the bite of infectious mosquitoes, either Aedes aegypti or Aedes albopictus. There are 4 distinct types of dengue virus, called DEN-1, DEN-2, DEN-3 and DEN-4. Dengue can be mild, as in most cases, or severe, as in few cases. It can cause serious illness and death among children. In Myanmar, July to August is peak season for transmission of dengue - coinciding with monsoon. How every one of us can help prevent dengue source: VBDC, Department of Public Health, Ministry of Health & Sports, 2019 z community awareness for mosquito breeding As shown above, dengue is cyclical in Myanmar, control is key alternating annually, as is the case in many countries. z cover, empty and clean domestic water storage At the same time, the number of dengue cases, and containers -- weekly is best deaths due to denge, over the period 2015 to 2018, z remove from the environment plastic bottles, both show a decreasing trend overall. plastic bags, fruit cans, discarded tyres -- for these are preferred mosquito breeding sites by Examples of how national and local authorities aedes aegypti (depicted) and aedes albopictus contribute z drain water collection points around the house z providing support for dengue management at z seek advice of a health professional if you have health facilities. multiple signs or symptoms of dengue z strengthening online reporting of dengue, How to recognize dengue? what are signs and using electronic based information system. -
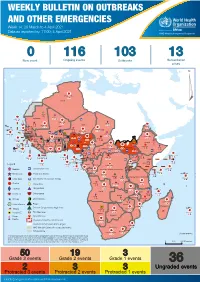
Weekly Bulletin on Outbreaks
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 14: 29 March to 4 April 2021 Data as reported by: 17:00; 4 April 2021 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 0 116 103 13 New event Ongoing events Outbreaks Humanitarian crises 117 622 3 105 Algeria ¤ 36 13 110 0 5 420 164 Mauritania 7 2 10 501 392 110 0 7 0 Niger 17 927 449 Mali 3 334 10 567 0 6 0 2 079 4 4 595 165 Eritrea Cape Verde 38 520 1 037 Chad Senegal 4 918 185 59 0 Gambia 27 0 3 0 17 125 159 9 761 45 Guinea-Bissau 796 17 7 0 Burkina Faso 225 46 215 189 2 963 0 162 593 2 048 Guinea 12 817 150 12 38 397 1 3 662 66 1 1 23 12 Benin 30 0 Nigeria 1 873 71 0 Ethiopia 420 14 481 5 6 188 15 Sierra Leone Togo 3 473 296 53 920 779 52 14 Ghana 5 245 72 Côte d'Ivoire 10 098 108 14 484 479 63 0 40 0 Liberia 17 0 South Sudan Central African Republic 916 2 45 0 25 0 19 670 120 43 180 237 90 287 740 Cameroon 7 0 28 676 137 5 330 13 138 988 2 224 1 952 87 655 2 51 22 43 0 112 12 6 1 488 6 3 988 79 11 187 6 902 102 Equatorial Guinea Uganda 542 8 Sao Tome and Principe 32 11 2 042 85 41 016 335 Kenya Legend 7 100 90 Gabon Congo 18 504 301 Rwanda Humanitarian crisis 2 212 34 22 482 311 Measles 18 777 111 Democratic Republic of the Congo 9 681 135 Burundi 2 964 6 Monkeypox Ebola virus disease Seychelles 27 930 739 1 525 0 420 29 United Republic of Tanzania Lassa fever Skin disease of unknown etiology 189 0 4 084 20 509 21 Cholera Yellow fever 1 349 5 6 257 229 22 631 542 cVDPV2 Dengue fever 88 930 1 220 Comoros Angola Malawi COVID-19 Chikungunya 33 661 1 123 862 0 3 719 146 -

Lora Grainger Dissertation 6 23 10
Characterization of the Transcripts that Encode pUL138, a Latency Determinant, During Human Cytomegalovirus Infection Item Type text; Electronic Dissertation Authors Grainger, Lora Ann Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 24/09/2021 15:04:11 Link to Item http://hdl.handle.net/10150/195915 1 CHARACTERIZATION OF THE TRANSCRIPTS THAT ENCODE PUL138, A LATENCY DETERMINANT, DURING HUMAN CYTOMEGALOVIRUS INFECTION by Lora A. Grainger Copyright © Lora A. Grainger 2010 A Dissertation Submitted to the Faculty of the DEPARTMENT OF IMMUNOBIOLOGY In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2010 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Lora A. Grainger entitled: Characterization of the Transcripts that Encode pUL138, a Latency Determinant, During Human Cytomegalovirus Infection. We recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy. _____________________________________________________Date: 6/15/10 Dr. Nafees Ahmad _____________________________________________________ Date: 6/15/10 Dr. Lonnie Lybarger _____________________________________________________ Date: 6/15/10 Dr. Carol Dieckmann Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement _____________________________________________________Date: 6/15/10 Dissertation Director: Dr. -

Holmes Washington 0250E 22
©Copyright 2020 Daniel Holmes Identification of Targetable Vulnerabilities During Latent KSHV Infection Daniel Holmes A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2020 Reading Committee: Michael Lagunoff, Chair Adam Philip Geballe Jason G Smith Program Authorized to Offer Degree: Department of Microbiology University of Washington Abstract Identification of Targetable Vulnerabilities During Latent KSHV Infection Daniel Holmes Chair of the Supervisory Committee: Professor Michael Lagunoff Department of Microbiology Viruses are defined as obligate intracellular parasites that require host processes to repli- cate. Latent virus life cycles are no exception to this definition, as viruses are still reliant on host machinery for continued proliferation and maintenance of viral genomes, even in the absence of lytic replication. In this thesis, I used essentiality screening to identify host factors on which Kaposi's Sarcoma Associated Herpesvirus (KSHV) relies for the proliferation and survival of latently infected cells. KSHV is the etiological agent of Kaposi's Sarcoma (KS), an endothelial cell-based tumor where more than 90% of the endothelial cells in the tumor are latently infected with KSHV. While traditional therapies for herpesviruses target lytic replication, the prevalence of latency in KS necessitates exploration of options for intervening in this stage of the viral life cycle. I performed CRISPR/Cas9 screening using lentiviral vec- tors encoding a library of single guide RNAs (sgRNAs) targeting every protein coding gene in the human genome. I compared mock infected and KSHV infected endothelial cells eight days post infection to identify genes essential to latent KSHV infection. -

For Viperin/Cig5 Response in Human Fetal Astrocytes
TLR3 Ligation Activates an Antiviral Response in Human Fetal Astrocytes: A Role for Viperin/cig5 This information is current as Mark A. Rivieccio, Hyeon-Sook Suh, Yongmei Zhao, of October 1, 2021. Meng-Liang Zhao, Keh Chuang Chin, Sunhee C. Lee and Celia F. Brosnan J Immunol 2006; 177:4735-4741; ; doi: 10.4049/jimmunol.177.7.4735 http://www.jimmunol.org/content/177/7/4735 Downloaded from References This article cites 40 articles, 16 of which you can access for free at: http://www.jimmunol.org/content/177/7/4735.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 1, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2006 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology TLR3 Ligation Activates an Antiviral Response in Human Fetal Astrocytes: A Role for Viperin/cig51 Mark A. Rivieccio,2,3* Hyeon-Sook Suh,2* Yongmei Zhao,* Meng-Liang Zhao,* Keh Chuang Chin,‡ Sunhee C. -

Chikungunya Annual Report 2017
Chikungunya Annual Report 2017 Chikungunya Chikungunya is a class B disease. Class B diseases or conditions shall be reported to the Office of Public Health by the end of the next business day after the existence of a case, a suspected case, or a positive la- boratory result is known Chikungunya is caused by a mosquito-borne alphavirus indigenous to tropical Africa and Asia, where it causes endemic and epidemic chikungunya fever. Chikungunya is a Makonde word (one of the local lan- guages in Tanzania) meaning ‘that which bends up’, and describes the symptoms caused by the severe joint pains that usually accompany the infection. Diseases range from silent asymptomatic infections to undifferentiated febrile illness and to devastating encephalitis. Deaths are rare. Fatal cases have not been documented conclusively. Chikungunya belongs to the Arbovirus group; it is transmitted by Aedes mosquitoes. The virus multiplies in a mosquito within eight to ten days; the mosquito remains infective for life. Viremic humans are the main reservoir for the virus. The mosquito becomes infected by feeding on viremic patients from one day before, to the last day of the febrile period. The virus is transmitted during probing and blood feeding. There is no person-to-person transmission. Vertical transmission from mother to fetus is rare; transplacental chikungunya transmission and severe congenital chikungunya has been described. In Africa, the virus is maintained in the sylvatic cycle between wild primates and forest-dwelling Aedes. Serological studies have found antibodies in humans and wild primates in the moist forests and semi- arid savannas of Africa. There are 27 alphaviruses able to infect vertebrates (humans, rodents, birds, horses) larger mammals and invertebrates.