CDC Chikungunya Virus Diagnostic Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Chikungunya Fever: Epidemiology, Clinical Syndrome, Pathogenesis
Antiviral Research 99 (2013) 345–370 Contents lists available at SciVerse ScienceDirect Antiviral Research journal homepage: www.elsevier.com/locate/antiviral Review Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy ⇑ Simon-Djamel Thiberville a,b, , Nanikaly Moyen a,b, Laurence Dupuis-Maguiraga c,d, Antoine Nougairede a,b, Ernest A. Gould a,b, Pierre Roques c,d, Xavier de Lamballerie a,b a UMR_D 190 ‘‘Emergence des Pathologies Virales’’ (Aix-Marseille Univ. IRD French Institute of Research for Development EHESP French School of Public Health), Marseille, France b University Hospital Institute for Infectious Disease and Tropical Medicine, Marseille, France c CEA, Division of Immuno-Virologie, Institute of Emerging Diseases and Innovative Therapies, Fontenay-aux-Roses, France d UMR E1, University Paris Sud 11, Orsay, France article info abstract Article history: Chikungunya virus (CHIKV) is the aetiological agent of the mosquito-borne disease chikungunya fever, a Received 7 April 2013 debilitating arthritic disease that, during the past 7 years, has caused immeasurable morbidity and some Revised 21 May 2013 mortality in humans, including newborn babies, following its emergence and dispersal out of Africa to the Accepted 18 June 2013 Indian Ocean islands and Asia. Since the first reports of its existence in Africa in the 1950s, more than Available online 28 June 2013 1500 scientific publications on the different aspects of the disease and its causative agent have been pro- duced. Analysis of these publications shows that, following a number of studies in the 1960s and 1970s, Keywords: and in the absence of autochthonous cases in developed countries, the interest of the scientific commu- Chikungunya virus nity remained low. -

Antibody-Mediated Enhancement Aggravates Chikungunya Virus
www.nature.com/scientificreports OPEN Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity Received: 14 July 2017 Fok-Moon Lum 1,2, Thérèse Couderc3,4, Bing-Shao Chia1,8, Ruo-Yan Ong1,9, Zhisheng Her1,10, Accepted: 17 January 2018 Angela Chow5, Yee-Sin Leo5, Yiu-Wing Kam1, Laurent Rénia1, Marc Lecuit 3,4,6 & Published: xx xx xxxx Lisa F. P. Ng1,2,7 The arthropod-transmitted chikungunya virus (CHIKV) causes a fu-like disease that is characterized by incapacitating arthralgia. The re-emergence of CHIKV and the continual risk of new epidemics have reignited research in CHIKV pathogenesis. Virus-specifc antibodies have been shown to control virus clearance, but antibodies present at sub-neutralizing concentrations can also augment virus infection that exacerbates disease severity. To explore this occurrence, CHIKV infection was investigated in the presence of CHIKV-specifc antibodies in both primary human cells and a murine macrophage cell line, RAW264.7. Enhanced attachment of CHIKV to the primary human monocytes and B cells was observed while increased viral replication was detected in RAW264.7 cells. Blocking of specifc Fc receptors (FcγRs) led to the abrogation of these observations. Furthermore, experimental infection in adult mice showed that animals had higher viral RNA loads and endured more severe joint infammation in the presence of sub-neutralizing concentrations of CHIKV-specifc antibodies. In addition, CHIKV infection in 11 days old mice under enhancing condition resulted in higher muscles viral RNA load detected and death. These observations provide the frst evidence of antibody-mediated enhancement in CHIKV infection and pathogenesis and could also be relevant for other important arboviruses such as Zika virus. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -

Prevent Dengue & Chikungunya
Prevent Dengue & Chikungunya WHO Myanmar newsletter special, 9 September 2019 What is dengue? Dengue situation in Myanmar Dengue is one of the most common mosquito- Cases and deaths 2015-2019 borne viral infections. The infection is spread from one person to another through the bite of infectious mosquitoes, either Aedes aegypti or Aedes albopictus. There are 4 distinct types of dengue virus, called DEN-1, DEN-2, DEN-3 and DEN-4. Dengue can be mild, as in most cases, or severe, as in few cases. It can cause serious illness and death among children. In Myanmar, July to August is peak season for transmission of dengue - coinciding with monsoon. How every one of us can help prevent dengue source: VBDC, Department of Public Health, Ministry of Health & Sports, 2019 z community awareness for mosquito breeding As shown above, dengue is cyclical in Myanmar, control is key alternating annually, as is the case in many countries. z cover, empty and clean domestic water storage At the same time, the number of dengue cases, and containers -- weekly is best deaths due to denge, over the period 2015 to 2018, z remove from the environment plastic bottles, both show a decreasing trend overall. plastic bags, fruit cans, discarded tyres -- for these are preferred mosquito breeding sites by Examples of how national and local authorities aedes aegypti (depicted) and aedes albopictus contribute z drain water collection points around the house z providing support for dengue management at z seek advice of a health professional if you have health facilities. multiple signs or symptoms of dengue z strengthening online reporting of dengue, How to recognize dengue? what are signs and using electronic based information system. -
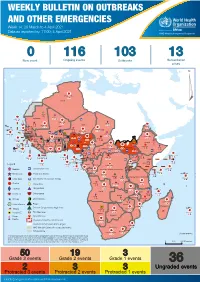
Weekly Bulletin on Outbreaks
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 14: 29 March to 4 April 2021 Data as reported by: 17:00; 4 April 2021 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 0 116 103 13 New event Ongoing events Outbreaks Humanitarian crises 117 622 3 105 Algeria ¤ 36 13 110 0 5 420 164 Mauritania 7 2 10 501 392 110 0 7 0 Niger 17 927 449 Mali 3 334 10 567 0 6 0 2 079 4 4 595 165 Eritrea Cape Verde 38 520 1 037 Chad Senegal 4 918 185 59 0 Gambia 27 0 3 0 17 125 159 9 761 45 Guinea-Bissau 796 17 7 0 Burkina Faso 225 46 215 189 2 963 0 162 593 2 048 Guinea 12 817 150 12 38 397 1 3 662 66 1 1 23 12 Benin 30 0 Nigeria 1 873 71 0 Ethiopia 420 14 481 5 6 188 15 Sierra Leone Togo 3 473 296 53 920 779 52 14 Ghana 5 245 72 Côte d'Ivoire 10 098 108 14 484 479 63 0 40 0 Liberia 17 0 South Sudan Central African Republic 916 2 45 0 25 0 19 670 120 43 180 237 90 287 740 Cameroon 7 0 28 676 137 5 330 13 138 988 2 224 1 952 87 655 2 51 22 43 0 112 12 6 1 488 6 3 988 79 11 187 6 902 102 Equatorial Guinea Uganda 542 8 Sao Tome and Principe 32 11 2 042 85 41 016 335 Kenya Legend 7 100 90 Gabon Congo 18 504 301 Rwanda Humanitarian crisis 2 212 34 22 482 311 Measles 18 777 111 Democratic Republic of the Congo 9 681 135 Burundi 2 964 6 Monkeypox Ebola virus disease Seychelles 27 930 739 1 525 0 420 29 United Republic of Tanzania Lassa fever Skin disease of unknown etiology 189 0 4 084 20 509 21 Cholera Yellow fever 1 349 5 6 257 229 22 631 542 cVDPV2 Dengue fever 88 930 1 220 Comoros Angola Malawi COVID-19 Chikungunya 33 661 1 123 862 0 3 719 146 -

Chikungunya Annual Report 2017
Chikungunya Annual Report 2017 Chikungunya Chikungunya is a class B disease. Class B diseases or conditions shall be reported to the Office of Public Health by the end of the next business day after the existence of a case, a suspected case, or a positive la- boratory result is known Chikungunya is caused by a mosquito-borne alphavirus indigenous to tropical Africa and Asia, where it causes endemic and epidemic chikungunya fever. Chikungunya is a Makonde word (one of the local lan- guages in Tanzania) meaning ‘that which bends up’, and describes the symptoms caused by the severe joint pains that usually accompany the infection. Diseases range from silent asymptomatic infections to undifferentiated febrile illness and to devastating encephalitis. Deaths are rare. Fatal cases have not been documented conclusively. Chikungunya belongs to the Arbovirus group; it is transmitted by Aedes mosquitoes. The virus multiplies in a mosquito within eight to ten days; the mosquito remains infective for life. Viremic humans are the main reservoir for the virus. The mosquito becomes infected by feeding on viremic patients from one day before, to the last day of the febrile period. The virus is transmitted during probing and blood feeding. There is no person-to-person transmission. Vertical transmission from mother to fetus is rare; transplacental chikungunya transmission and severe congenital chikungunya has been described. In Africa, the virus is maintained in the sylvatic cycle between wild primates and forest-dwelling Aedes. Serological studies have found antibodies in humans and wild primates in the moist forests and semi- arid savannas of Africa. There are 27 alphaviruses able to infect vertebrates (humans, rodents, birds, horses) larger mammals and invertebrates. -

Chikungunya Virus and Its Mosquito Vectors
VECTOR-BORNE AND ZOONOTIC DISEASES Volume 15, Number 4, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/vbz.2014.1745 Chikungunya Virus and Its Mosquito Vectors Stephen Higgs1,2 and Dana Vanlandingham1,2 Abstract Chikungunya virus (CHIKV), a mosquito-borne alphavirus of increasing public health significance, has caused large epidemics in Africa and the Indian Ocean basin; now it is spreading throughout the Americas. The primary vectors of CHIKV are Aedes (Ae.) aegypti and, after the introduction of a mutation in the E1 envelope protein gene, the highly anthropophilic and geographically widespread Ae. albopictus mosquito. We review here research efforts to characterize the viral genetic basis of mosquito–vector interactions, the use of RNA interference and other strategies for the control of CHIKV in mosquitoes, and the potentiation of CHIKV infection by mosquito saliva. Over the past decade, CHIKV has emerged on a truly global scale. Since 2013, CHIKV transmission has been reported throughout the Caribbean region, in North America, and in Central and South American countries, including Brazil, Columbia, Costa Rica, El Salvador, French Guiana, Guatemala, Guyana, Nicaragua, Panama, Suriname, and Venezuela. Closing the gaps in our knowledge of driving factors behind the rapid geographic expansion of CHIKV should be considered a research priority. The abundance of multiple primate species in many of these countries, together with species of mosquito that have never been exposed to CHIKV, may provide opportunities for this highly adaptable virus to establish sylvatic cycles that to date have not been seen outside of Africa. The short-term and long-term ecological consequences of such transmission cycles, including the impact on wildlife and people living in these areas, are completely unknown. -

Preparedness and Response for Chikungunya Virus: Introduction in the Americas Washington, D.C.: PAHO, © 2011
O P S N N O PR O V United States of America Washington, D.C. 20037, 525 Twenty-third Street, N.W., I S M A U L U N T D E I O H P A PAHO/CDC PREparEDNESS AND RESPONSE FOR CHIKUNGUNYA VIRUS INTRODUCTION IN THE AMERICAS Chikungunya Virus Chikungunya Introduction in theAmericas in Introduction Preparedness andResponse for Preparedness and Response for Chikungunya Virus Introduction in the Americas S A LU O T R E P O P A P H S O N I O D V I M U N PAHO HQ Library Cataloguing-in-Publication Pan American Health Organization Preparedness and Response for Chikungunya Virus: Introduction in the Americas Washington, D.C.: PAHO, © 2011 ISBN: 978-92-75-11632-6 I. Title 1. VECTOR CONTROL 2. COMMUNICABLE DISEASE CONTROL 3. EPIDEMIOLOGIC SURVEILLANCE 4. DISEASE OUTBREAKS 5. VIRUS DISEASE - transmission 6. LABORATORY TECHNIQUES AND PROCEDURES 7. AMERICAS NLM QX 650.DA1 The Pan American Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and inquiries should be addressed to Editorial Services, Area of Knowledge Management and Communications (KMC), Pan American Health Organization, Washington, D.C., U.S.A. The Area for Health Surveillance and Disease Prevention and Control, Project for Alert and Response and Epidemic Diseases, at (202) 974-3010 or via email at [email protected], will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. ©Pan American Health Organization, 2011. -

Is It Chikungunya Or Dengue? Chikungunya and Dengue Are Viruses Most Commonly Transmitted by Aedes Aegypti and Aedes Albopictus Mosquitoes
Is it Chikungunya or Dengue? Chikungunya and dengue are viruses most commonly transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Anyone who lives in or has traveled to an area where chikungunya or dengue viruses are found is at risk for infection. Chikungunya virus Dengue virus Treatment for Chikungunya and Dengue • Approximately 3 in 4 people infected • Approximately 1 in 4 people infected will develop disease. Treat symptoms: Assess hydration and hemodynamic status and provide will develop disease. • Incubation: 4–7 days (range: 3–14 days) supportive care as needed. If adequate, instruct the patient to rest and S M T W T F S drink fluids. • Incubation: 3–7 days (range: 2–12 days) • Some patients may develop life threatening consequences and • Acute symptoms typically resolve within 7-10 days. require hospitalization. Treat or manage other conditions: Evaluate for other serious conditions S M T W T F S S M T W T F S (e.g., malaria and bacterial infections). • Persons at risk for severe disease include neonates • Since there are 4 distinct dengue viruses, a person can be exposed intrapartum, older adults, and persons with infected up to 4 times. Infection with each dengue virus type Relieve fever and pain: Recommend acetaminophen or paracetamol until underlying medical conditions. confers lifetime immunity for that specific virus type. dengue is ruled out. Aspirin and NSAIDs (like ibuprofen) can increase risk of bleeding in patients with dengue. NSAIDs, corticosteroids, or physiotherapy • Infection is thought to confer lifetime immunity. • Risk areas: http://www.cdc.gov/dengue/ may help treat persistent joint pain. -

WEEKLY BULLETIN on OUTBREAKS and OTHER EMERGENCIES Week 12: 16 - 22 March 2020 Data As Reported By: 17:00; 22 March 2020
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 12: 16 - 22 March 2020 Data as reported by: 17:00; 22 March 2020 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 7 95 91 11 New events Ongoing events Outbreaks Humanitarian crises 201 17 Algeria 1 0 91 0 2 0 Gambia 1 0 1 0 Mauritania 14 7 20 0 9 0 Senegal 304 1 1 0Eritrea Niger 2 410 23 Mali 67 0 1 0 3 0 Burkina Faso 41 7 1 0 Cabo Verdé Guinea Chad 1 251 0 75 3 53 0 4 0 4 690 18 4 1 22 0 21 0 Nigeria 2 0 Côte d’Ivoire 1 873 895 15 4 0 South Sudan 917 172 40 0 3 970 64 Ghana16 0 139 0 186 3 1 0 14 0 Liberia 25 0 Central African Benin Cameroon 19 0 4 732 26 Ethiopia 24 0 Republic Togo 1 618 5 7 626 83 352 14 1 449 71 2 1 Uganda 36 16 Democratic Republic 637 1 169 0 9 0 15 0 Equatorial of Congo 15 5 202 0 Congo 1 0 Guinea 6 0 3 453 2 273 Kenya 1 0 253 1 Legend 3 0 6 0 38 0 37 0 Gabon 29 981 384 Rwanda 21 0 Measles Humanitarian crisis 2 0 4 0 4 998 63 Burundi 7 0 Hepatitis E 8 0 Monkeypox 8 892 300 3 294 Seychelles 30 2 108 0 Tanzania Yellow fever 12 0 Lassa fever 79 0 Dengue fever Cholera Angola 547 14 Ebola virus disease Rift Valley Fever Comoros 129 0 2 0 Chikungunya Malawi 218 0 cVDPV2 2 0 Zambia Leishmaniasis Mozambique 3 0 3 0 COVID-19 Plague Zimbabwe 313 13 Madagascar Anthrax Crimean-Congo haemorrhagic fever Namibia 286 1 Malaria 2 0 24 2 12 0 Floods Meningitis 3 0 Mauritius Cases 7 063 59 1 0 Deaths Countries reported in the document Non WHO African Region Eswatini N WHO Member States with no reported events W E 3 0 Lesotho4 0 402 0 South Africa 20 0 S South Africa Graded events † 40 15 1 Grade 3 events Grade 2 events Grade 1 events 39 22 20 31 Ungraded events ProtractedProtracted 3 3 events events Protracted 2 events ProtractedProtracted 1 1 events event Health Emergency Information and Risk Assessment Overview This Weekly Bulletin focuses on public health emergencies occurring in the WHO Contents African Region. -

Suramin Is a Potent Inhibitor of Chikungunya and Ebola Virus Cell
Henß et al. Virology Journal (2016) 13:149 DOI 10.1186/s12985-016-0607-2 RESEARCH Open Access Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry Lisa Henß1, Simon Beck1, Tatjana Weidner1, Nadine Biedenkopf2,3, Katja Sliva1, Christopher Weber1, Stephan Becker2,3 and Barbara S. Schnierle1* Abstract Background: Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus that causes high fever, rash, and recurrent arthritis in humans. It has efficiently adapted to Aedes albopictus, which also inhabits temperate regions and currently causes large outbreaks in the Caribbean and Latin America. Ebola virus (EBOV) is a member of the filovirus family. It causes the Ebola virus disease (EDV), formerly known as Ebola hemorrhagic fever in humans and has a mortality rate of up to 70 %. The last outbreak in Western Africa was the largest in history and has caused approximately 25,000 cases and 10,000 deaths. For both viral infections no specific treatment or licensed vaccine is currently available. The bis-hexasulfonated naphthylurea, suramin, is used as a treatment for trypanosome-caused African river blindness. As a competitive inhibitor of heparin, suramin has been described to have anti-viral activity. Methods: We tested the activity of suramin during CHIKV or Ebola virus infection, using CHIKV and Ebola envelope glycoprotein pseudotyped lentiviral vectors and wild-type CHIKV and Ebola virus. Results: Suramin efficiently inhibited CHIKV and Ebola envelope-mediated gene transfer while vesicular stomatitis virus G protein pseudotyped vectors were only marginally affected. In addition, suramin was able to inhibit wild-type CHIKV and Ebola virus replication in vitro.