Federal Register/Vol. 83, No. 165/Friday, August 24, 2018/Notices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Uveal Involvement in Marburg Virus Disease B
Br J Ophthalmol: first published as 10.1136/bjo.61.4.265 on 1 April 1977. Downloaded from British Journal of Ophthalmology, 1977, 61, 265-266 Uveal involvement in Marburg virus disease B. S. KUMING AND N. KOKORIS From the Department of Ophthalmology, Johannesburg General Hospital and University of the Witwatersrand SUMMARY The first reported case of uveal involvement in Marburg virus disease is described. 'Ex Africa semper aliquid novi'. Two outbreaks of Marburg virus disease have been Rhodesia and had also been constantly at his documented. The first occurred in Marburg and bedside till his death. Lassa fever was suspected and Frankfurt, West Germany, in 1967 (Martini, 1969) she was given a unit of Lassa fever convalescent and the second in Johannesburg in 1975 (Gear, serum when she became desperately ill on the fifth 1975). This case report describes the third patient day. She also developed acute pancreatitis. Within in the Johannesburg outbreak, who developed an 52 hours she made a dramatic and uneventful anterior uveitis. The cause of the uveitis was proved recovery. Her illness mainly affected the haema- to be the Marburg virus by identiying it in a tissue topoietic, hepatic, and pancreatic systems. culture of her aqueous fluid. The subject of this report was a nurse who had helped to nurse patients 1 and 2. Nine days after the Case report death ofthe first patient she presented with lower back pain and high fever. She developed hepatitis, a mild Before describing the case history of the patient the disseminated intravascular coagulation syndrome, events leading to her contracting the disease must successfully treated with heparin, and the classical be briefly described. -

Chikungunya Fever: Epidemiology, Clinical Syndrome, Pathogenesis
Antiviral Research 99 (2013) 345–370 Contents lists available at SciVerse ScienceDirect Antiviral Research journal homepage: www.elsevier.com/locate/antiviral Review Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy ⇑ Simon-Djamel Thiberville a,b, , Nanikaly Moyen a,b, Laurence Dupuis-Maguiraga c,d, Antoine Nougairede a,b, Ernest A. Gould a,b, Pierre Roques c,d, Xavier de Lamballerie a,b a UMR_D 190 ‘‘Emergence des Pathologies Virales’’ (Aix-Marseille Univ. IRD French Institute of Research for Development EHESP French School of Public Health), Marseille, France b University Hospital Institute for Infectious Disease and Tropical Medicine, Marseille, France c CEA, Division of Immuno-Virologie, Institute of Emerging Diseases and Innovative Therapies, Fontenay-aux-Roses, France d UMR E1, University Paris Sud 11, Orsay, France article info abstract Article history: Chikungunya virus (CHIKV) is the aetiological agent of the mosquito-borne disease chikungunya fever, a Received 7 April 2013 debilitating arthritic disease that, during the past 7 years, has caused immeasurable morbidity and some Revised 21 May 2013 mortality in humans, including newborn babies, following its emergence and dispersal out of Africa to the Accepted 18 June 2013 Indian Ocean islands and Asia. Since the first reports of its existence in Africa in the 1950s, more than Available online 28 June 2013 1500 scientific publications on the different aspects of the disease and its causative agent have been pro- duced. Analysis of these publications shows that, following a number of studies in the 1960s and 1970s, Keywords: and in the absence of autochthonous cases in developed countries, the interest of the scientific commu- Chikungunya virus nity remained low. -

Antibody-Mediated Enhancement Aggravates Chikungunya Virus
www.nature.com/scientificreports OPEN Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity Received: 14 July 2017 Fok-Moon Lum 1,2, Thérèse Couderc3,4, Bing-Shao Chia1,8, Ruo-Yan Ong1,9, Zhisheng Her1,10, Accepted: 17 January 2018 Angela Chow5, Yee-Sin Leo5, Yiu-Wing Kam1, Laurent Rénia1, Marc Lecuit 3,4,6 & Published: xx xx xxxx Lisa F. P. Ng1,2,7 The arthropod-transmitted chikungunya virus (CHIKV) causes a fu-like disease that is characterized by incapacitating arthralgia. The re-emergence of CHIKV and the continual risk of new epidemics have reignited research in CHIKV pathogenesis. Virus-specifc antibodies have been shown to control virus clearance, but antibodies present at sub-neutralizing concentrations can also augment virus infection that exacerbates disease severity. To explore this occurrence, CHIKV infection was investigated in the presence of CHIKV-specifc antibodies in both primary human cells and a murine macrophage cell line, RAW264.7. Enhanced attachment of CHIKV to the primary human monocytes and B cells was observed while increased viral replication was detected in RAW264.7 cells. Blocking of specifc Fc receptors (FcγRs) led to the abrogation of these observations. Furthermore, experimental infection in adult mice showed that animals had higher viral RNA loads and endured more severe joint infammation in the presence of sub-neutralizing concentrations of CHIKV-specifc antibodies. In addition, CHIKV infection in 11 days old mice under enhancing condition resulted in higher muscles viral RNA load detected and death. These observations provide the frst evidence of antibody-mediated enhancement in CHIKV infection and pathogenesis and could also be relevant for other important arboviruses such as Zika virus. -

Viral Hemorrhagic Fevers and Bioterrorism
What you need to know about . Viral Hemorrhagic Fevers and Bioterrorism What are viral hemorrhagic fevers? How are viral hemorrhagic fevers Viral hemorrhagic fevers (VHFs) are a spread? group of illnesses caused by several distinct In nature, viruses causing hemorrhagic fever families of viruses. In general the term typically are passed from mice, rats, fleas “viral hemorrhagic fever” describes severe and ticks to humans. People can be infected problems affecting several organ systems when they come in contact with urine, fecal in the body. Typically, the entire system of ma�er, saliva or other body fluids from blood vessels is damaged, and the body has infected rodents. Fleas and ticks transmit the problems regulating itself. Symptoms o�en viruses when they bite a person or when a include bleeding, but the bleeding itself is person crushes a tick. Hosts for some viruses rarely life-threatening. VHFs are caused by such as Ebola and Marburg are not known. viruses of four families: Some viruses such as Ebola, Marburg and Lassa can be spread from person to person • Arenavirus including Lassa fever and by direct contact with infected blood or Argentine, Bolivian, Brazilian and organs or indirectly through contact with Venezuelan hemorrhagic fevers; objects such as syringes or needles that are • Filovirus including Ebola and Marburg; contaminated with infected body fluids. • Bunyavirus including Hantavirus and Ri� Valley Fever; What are the symptoms? • Flavivirus including yellow fever and Symptoms vary with the different virus dengue fever. families, but first signs o�en include sudden fever, weakness, muscle pain, tiredness, Can viral hemorrhagic fevers be used headache and sore throat. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -

Viral Hemorrhagic Fevers (Lassa, Marburg, Ebola, Crimean-Congo, and Other Emerging Viruses)
Viral Hemorrhagic Fevers (Lassa, Marburg, Ebola, Crimean-Congo, and other emerging viruses) What Are They? Viral hemorrhagic fevers are a group of illnesses causes by several viruses. These viruses affect multiple organs in the body by damaging the vascular (blood vessel) system. The bleeding or hemorrhaging caused by the virus is not usually life threatening but damage to organ systems in the body can range from mild to deadly. The viruses responsible for this type of illness include Lassa, Marburg, Ebola, and Crimean-Congo hemorrhagic fever. How can you get it? These emerging viral hemorrhagic fevers are presumed to be animal borne (zoonotic) and can be transmitted to humans through contact. Infected humans can spread the virus to each other through contact with contaminated objects or blood. The risk of acquiring these diseases is typically restricted to the geographic regions where the virus is found. Given global travel, rare cases have been reported outside of the host region. These rare cases are probably the greatest form of the occupational threat to fire fighters. Lassa Associated with specific rodents Found in West Africa Marburg Transmitted by African fruit bat Found in Africa Ebola Transmitted by unknown animal Found in Africa Crimean-Congo Tick-borne virus Found in Africa, Asia, Europe What are the symptoms? The time to develop symptoms varies by virus but is between 2 to 21 days after exposure to the Ebola virus. The signs and symptoms of viral hemorrhagic fever vary depending on the virus but include: Flu-like symptoms o Fever o Fatigue o Muscle aches Exhaustion Nausea and/or vomiting Abdominal pain Shock Seizures Delirium Bleeding Organ failure The most common complication of Lassa fever is deafness. -

1 Lujo Viral Hemorrhagic Fever: Considering Diagnostic Capacity And
1 Lujo Viral Hemorrhagic Fever: Considering Diagnostic Capacity and 2 Preparedness in the Wake of Recent Ebola and Zika Virus Outbreaks 3 4 Dr Edgar Simulundu1,, Prof Aaron S Mweene1, Dr Katendi Changula1, Dr Mwaka 5 Monze2, Dr Elizabeth Chizema3, Dr Peter Mwaba3, Prof Ayato Takada1,4,5, Prof 6 Guiseppe Ippolito6, Dr Francis Kasolo7, Prof Alimuddin Zumla8,9, Dr Matthew Bates 7 8,9,10* 8 9 1 Department of Disease Control, School of Veterinary Medicine, University of Zambia, 10 Lusaka, Zambia 11 2 University Teaching Hospital & National Virology Reference Laboratory, Lusaka, Zambia 12 3 Ministry of Health, Republic of Zambia 13 4 Division of Global Epidemiology, Hokkaido University Research Center for Zoonosis 14 Control, Sapporo, Japan 15 5 Global Institution for Collaborative Research and Education, Hokkaido University, Sapporo, 16 Japan 17 6 Lazzaro Spallanzani National Institute for Infectious Diseases, IRCCS, Rome, Italy 18 7 World Health Organization, WHO Africa, Brazzaville, Republic of Congo 19 8 Department of Infection, Division of Infection and Immunity, University College London, 20 U.K 21 9 University of Zambia – University College London Research & Training Programme 22 (www.unza-uclms.org), University Teaching Hospital, Lusaka, Zambia 23 10 HerpeZ (www.herpez.org), University Teaching Hospital, Lusaka, Zambia 24 25 *Corresponding author: Dr. Matthew Bates 26 Address: UNZA-UCLMS Research & Training Programme, University Teaching Hospital, 27 Lusaka, Zambia, RW1X 1 28 Email: [email protected]; Phone: +260974044708 29 30 2 31 Abstract 32 Lujo virus is a novel old world arenavirus identified in Southern Africa in 2008 as the 33 cause of a viral hemorrhagic fever (VHF) characterized by nosocomial transmission 34 with a high case fatality rate of 80% (4/5 cases). -

Orthohantaviruses Belonging to Three Phylogroups All Inhibit Apoptosis in Infected Target Cells
www.nature.com/scientificreports OPEN Orthohantaviruses belonging to three phylogroups all inhibit apoptosis in infected target cells Received: 13 July 2018 Carles Solà-Riera1, Shawon Gupta1,2, Hans-Gustaf Ljunggren1 & Jonas Klingström 1 Accepted: 3 December 2018 Orthohantaviruses, previously known as hantaviruses, are zoonotic viruses that can cause hantavirus Published: xx xx xxxx pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS) in humans. The HPS-causing Andes virus (ANDV) and the HFRS-causing Hantaan virus (HTNV) have anti-apoptotic efects. To investigate if this represents a general feature of orthohantaviruses, we analysed the capacity of six diferent orthohantaviruses – belonging to three distinct phylogroups and representing both pathogenic and non-pathogenic viruses – to inhibit apoptosis in infected cells. Primary human endothelial cells were infected with ANDV, HTNV, the HFRS-causing Puumala virus (PUUV) and Seoul virus, as well as the putative non-pathogenic Prospect Hill virus and Tula virus. Infected cells were then exposed to the apoptosis-inducing chemical staurosporine or to activated human NK cells exhibiting a high cytotoxic potential. Strikingly, all orthohantaviruses inhibited apoptosis in both settings. Moreover, we show that the nucleocapsid (N) protein from all examined orthohantaviruses are potential targets for caspase-3 and granzyme B. Recombinant N protein from ANDV, PUUV and the HFRS-causing Dobrava virus strongly inhibited granzyme B activity and also, to certain extent, caspase-3 activity. Taken together, this study demonstrates that six diferent orthohantaviruses inhibit apoptosis, suggesting this to be a general feature of orthohantaviruses likely serving as a mechanism of viral immune evasion. Orthohantaviruses, of the order Bunyavirales and previously known as hantaviruses, are small single-stranded negative-sense RNA viruses with a tri-segmented genome (S, M and L segments) encoding four to fve proteins. -

Prevent Dengue & Chikungunya
Prevent Dengue & Chikungunya WHO Myanmar newsletter special, 9 September 2019 What is dengue? Dengue situation in Myanmar Dengue is one of the most common mosquito- Cases and deaths 2015-2019 borne viral infections. The infection is spread from one person to another through the bite of infectious mosquitoes, either Aedes aegypti or Aedes albopictus. There are 4 distinct types of dengue virus, called DEN-1, DEN-2, DEN-3 and DEN-4. Dengue can be mild, as in most cases, or severe, as in few cases. It can cause serious illness and death among children. In Myanmar, July to August is peak season for transmission of dengue - coinciding with monsoon. How every one of us can help prevent dengue source: VBDC, Department of Public Health, Ministry of Health & Sports, 2019 z community awareness for mosquito breeding As shown above, dengue is cyclical in Myanmar, control is key alternating annually, as is the case in many countries. z cover, empty and clean domestic water storage At the same time, the number of dengue cases, and containers -- weekly is best deaths due to denge, over the period 2015 to 2018, z remove from the environment plastic bottles, both show a decreasing trend overall. plastic bags, fruit cans, discarded tyres -- for these are preferred mosquito breeding sites by Examples of how national and local authorities aedes aegypti (depicted) and aedes albopictus contribute z drain water collection points around the house z providing support for dengue management at z seek advice of a health professional if you have health facilities. multiple signs or symptoms of dengue z strengthening online reporting of dengue, How to recognize dengue? what are signs and using electronic based information system. -
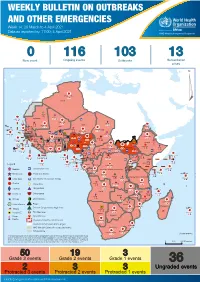
Weekly Bulletin on Outbreaks
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 14: 29 March to 4 April 2021 Data as reported by: 17:00; 4 April 2021 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 0 116 103 13 New event Ongoing events Outbreaks Humanitarian crises 117 622 3 105 Algeria ¤ 36 13 110 0 5 420 164 Mauritania 7 2 10 501 392 110 0 7 0 Niger 17 927 449 Mali 3 334 10 567 0 6 0 2 079 4 4 595 165 Eritrea Cape Verde 38 520 1 037 Chad Senegal 4 918 185 59 0 Gambia 27 0 3 0 17 125 159 9 761 45 Guinea-Bissau 796 17 7 0 Burkina Faso 225 46 215 189 2 963 0 162 593 2 048 Guinea 12 817 150 12 38 397 1 3 662 66 1 1 23 12 Benin 30 0 Nigeria 1 873 71 0 Ethiopia 420 14 481 5 6 188 15 Sierra Leone Togo 3 473 296 53 920 779 52 14 Ghana 5 245 72 Côte d'Ivoire 10 098 108 14 484 479 63 0 40 0 Liberia 17 0 South Sudan Central African Republic 916 2 45 0 25 0 19 670 120 43 180 237 90 287 740 Cameroon 7 0 28 676 137 5 330 13 138 988 2 224 1 952 87 655 2 51 22 43 0 112 12 6 1 488 6 3 988 79 11 187 6 902 102 Equatorial Guinea Uganda 542 8 Sao Tome and Principe 32 11 2 042 85 41 016 335 Kenya Legend 7 100 90 Gabon Congo 18 504 301 Rwanda Humanitarian crisis 2 212 34 22 482 311 Measles 18 777 111 Democratic Republic of the Congo 9 681 135 Burundi 2 964 6 Monkeypox Ebola virus disease Seychelles 27 930 739 1 525 0 420 29 United Republic of Tanzania Lassa fever Skin disease of unknown etiology 189 0 4 084 20 509 21 Cholera Yellow fever 1 349 5 6 257 229 22 631 542 cVDPV2 Dengue fever 88 930 1 220 Comoros Angola Malawi COVID-19 Chikungunya 33 661 1 123 862 0 3 719 146 -

Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation
Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation This lecture is on Ebola virus disease (EVD) and clinical care. This is part one of a three-part lecture on this topic. Preparing Healthcare Workers to Work in Ebola Treatment Units (ETUs) in Africa This lecture will focus on EVD in the West African setting. Ebola Virus Disease and Clinical Care: The training and information you receive in this course will Part I: History, Transmission, and Clinical not cover the use of certain interventions such as intubation Presentation or dialysis which are not available in West African Ebola Treatment Units (ETUs). You will need supplemental training This presentation is current as of December 2014. This presentation contains materials from Centers for Disease Control and to care for patients appropriately in countries where advanced Prevention (CDC), Médecins Sans Frontières (MSF), and World Health Organization (WHO). care is available. U.S. Department of Health and Human Services U.S. Department of Health and Human Services Centers for Disease Control and Prevention Centers for Disease Control and Prevention version 12.03.2014 The learning objectives for this lecture are to: Learning Objectives ▶ Describe the routes of Ebola virus transmission Describe the routes of Ebola virus transmission Explain when and how patients are infectious ▶ Explain when and how patients are infectious Describe the clinical features of patients with Ebola ▶ Describe screening criteria for Ebola virus disease Describe the clinical features of patients with Ebola (EVD) used in West Africa Explain how to identify patients with suspected ▶ Describe screening criteria for EVD used in West Africa EVD who present to the ETU ▶ Explain how to identify patients with suspected EVD who present to the ETU This presentation contains materials from CDC, MSF, and WHO 2 A number of different viruses cause viral hemorrhagic fever.