Dengue, Chikungunya, and Zika: Differences in Similarities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Executive Orders and Emergency Declarations for the West Nile Virus: Applying Lessons from Past Outbreaks to Zika
Executive Orders and Emergency Declarations for the West Nile Virus: Applying Lessons from Past Outbreaks to Zika Government leaders are often given the authority to issue executive orders (EOs), proclamations, or emergency declarations to address public health threats, such as that posed by the Zika virus.1 Local, state, and federal executive branch leaders have used these powers to address public health threats posed by other mosquito-borne diseases.2 While existing laws and regulations may allow localities, states, and the federal government to take action to combat mosquito-borne threats absent an EO or emergency declaration, examining such executive actions provides a snapshot of how some jurisdictions have responded to past outbreaks. As of February 21, 2016, only one territory and two states (Puerto Rico, Florida, and Hawaii) have issued emergency declarations that contemplate the threats posed by the Zika virus.3, 4 Historically, however, many US jurisdictions have taken such actions to address other mosquito-borne illnesses, such as West Nile virus. The following provides a brief analysis of select uses of local, state, and federal executive powers to combat West Nile virus. Examining the use of executive powers to address West 1 L Rutkow et al. The Public Health Workforce and Willingness to Respond to Emergencies: A 50-State Analysis of Potentially Influential Laws, 42 J. LAW MED. & ETHICS 64, 64 (2014) (“In the United States, at the federal, state, and local levels, laws provide an infrastructure for public health emergency preparedness and response efforts. Law is perhaps most visible during an emergency when the president or a state’s governor issues a disaster declaration establishing the temporal and geographic parameters for the response and making financial and other resources available.”). -

Asian Zika Virus Isolate Significantly Changes the Transcriptional Profile
viruses Article Asian Zika Virus Isolate Significantly Changes the Transcriptional Profile and Alternative RNA Splicing Events in a Neuroblastoma Cell Line Gaston Bonenfant 1,2, Ryan Meng 2, Carl Shotwell 2,3 , Pheonah Badu 1,2, Anne F. Payne 4, Alexander T. Ciota 4,5, Morgan A. Sammons 1, J. Andrew Berglund 1,2 and Cara T. Pager 1,2,* 1 Department of Biological Sciences, University at Albany-SUNY, Albany, NY 12222, USA; [email protected] (G.B.); [email protected] (P.B.); [email protected] (M.A.S.); [email protected] (J.A.B.) 2 The RNA Institute, University at Albany-SUNY, Albany, NY 12222, USA; [email protected] (R.M.); [email protected] (C.S.) 3 Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, FL 32610, USA 4 Wadsworth Center, New York State Department of Health (NYSDOH), Slingerlands, NY 12159, USA; [email protected] (A.F.P.); [email protected] (A.T.C.) 5 Department of Biomedical Sciences, University at Albany-SUNY, School of Public Health, Rensselaer, NY 12144, USA * Correspondence: [email protected]; Tel.: +1-518-591-8841 Received: 9 April 2020; Accepted: 27 April 2020; Published: 5 May 2020 Abstract: The alternative splicing of pre-mRNAs expands a single genetic blueprint to encode multiple, functionally diverse protein isoforms. Viruses have previously been shown to interact with, depend on, and alter host splicing machinery. The consequences, however, incited by viral infection on the global alternative slicing (AS) landscape are under-appreciated. Here, we investigated the transcriptional and alternative splicing profile of neuronal cells infected with a contemporary Puerto Rican Zika virus (ZIKVPR) isolate, an isolate of the prototypical Ugandan ZIKV (ZIKVMR), and dengue virus 2 (DENV2). -

Chikungunya Fever: Epidemiology, Clinical Syndrome, Pathogenesis
Antiviral Research 99 (2013) 345–370 Contents lists available at SciVerse ScienceDirect Antiviral Research journal homepage: www.elsevier.com/locate/antiviral Review Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy ⇑ Simon-Djamel Thiberville a,b, , Nanikaly Moyen a,b, Laurence Dupuis-Maguiraga c,d, Antoine Nougairede a,b, Ernest A. Gould a,b, Pierre Roques c,d, Xavier de Lamballerie a,b a UMR_D 190 ‘‘Emergence des Pathologies Virales’’ (Aix-Marseille Univ. IRD French Institute of Research for Development EHESP French School of Public Health), Marseille, France b University Hospital Institute for Infectious Disease and Tropical Medicine, Marseille, France c CEA, Division of Immuno-Virologie, Institute of Emerging Diseases and Innovative Therapies, Fontenay-aux-Roses, France d UMR E1, University Paris Sud 11, Orsay, France article info abstract Article history: Chikungunya virus (CHIKV) is the aetiological agent of the mosquito-borne disease chikungunya fever, a Received 7 April 2013 debilitating arthritic disease that, during the past 7 years, has caused immeasurable morbidity and some Revised 21 May 2013 mortality in humans, including newborn babies, following its emergence and dispersal out of Africa to the Accepted 18 June 2013 Indian Ocean islands and Asia. Since the first reports of its existence in Africa in the 1950s, more than Available online 28 June 2013 1500 scientific publications on the different aspects of the disease and its causative agent have been pro- duced. Analysis of these publications shows that, following a number of studies in the 1960s and 1970s, Keywords: and in the absence of autochthonous cases in developed countries, the interest of the scientific commu- Chikungunya virus nity remained low. -

An Overview of Mosquito Vectors of Zika Virus
Microbes and Infection xxx (2018) 1e15 Contents lists available at ScienceDirect Microbes and Infection journal homepage: www.elsevier.com/locate/micinf An overview of mosquito vectors of Zika virus Sebastien Boyer a, Elodie Calvez b, Thais Chouin-Carneiro c, Diawo Diallo d, * Anna-Bella Failloux e, a Institut Pasteur of Cambodia, Unit of Medical Entomology, Phnom Penh, Cambodia b Institut Pasteur of New Caledonia, URE Dengue and Other Arboviruses, Noumea, New Caledonia c Instituto Oswaldo Cruz e Fiocruz, Laboratorio de Transmissores de Hematozoarios, Rio de Janeiro, Brazil d Institut Pasteur of Dakar, Unit of Medical Entomology, Dakar, Senegal e Institut Pasteur, URE Arboviruses and Insect Vectors, Paris, France article info abstract Article history: The mosquito-borne arbovirus Zika virus (ZIKV, Flavivirus, Flaviviridae), has caused an outbreak Received 6 December 2017 impressive by its magnitude and rapid spread. First detected in Uganda in Africa in 1947, from where it Accepted 15 January 2018 spread to Asia in the 1960s, it emerged in 2007 on the Yap Island in Micronesia and hit most islands in Available online xxx the Pacific region in 2013. Subsequently, ZIKV was detected in the Caribbean, and Central and South America in 2015, and reached North America in 2016. Although ZIKV infections are in general asymp- Keywords: tomatic or causing mild self-limiting illness, severe symptoms have been described including neuro- Arbovirus logical disorders and microcephaly in newborns. To face such an alarming health situation, WHO has Mosquito vectors Aedes aegypti declared Zika as an emerging global health threat. This review summarizes the literature on the main fi Vector competence vectors of ZIKV (sylvatic and urban) across all the ve continents with special focus on vector compe- tence studies. -

Overview of Zika
Overview of Zika Alan D.T. Barrett Department of Pathology Sealy Center for Vaccine Development University of Texas Medical Branch Galveston TX Disclaimer • Over 700 papers in pubmed on Zika in the last 12 months. • Impossible to stay up to date as field is moving so fast. • Only including material in public domain. ZIKV: 1947-2006 • Zika virus (ZIKV) causes Zika fever (ZF), an acute febrile illness characterized by a rash, conjunctival injection, arthralgia, myalgia and headache. • The disease appears in all age groups with an incubation period on the order of 3-14 days and a symptomatic phase lasting about 2-7 days. • Treatment is largely symptomatic. • The illness is mild in nature with a very low rate of hospitalization. • The vast majority of patients make a full recovery and while death is rarely reported, it has primarily occurred in the immunocompromised or those with other complicating medical conditions. • Only 14 clinical cases in the literature from 1951-2006. Map showing the known distribution of Zika virus based on serosurveys, virus detection, and laboratory-diagnosed cases. Blue arrows show recent patterns of spread deduced from phylogenetic studies Weaver et al Antiviral Research, Volume 130, 2016, 69–80 ZIKV in the Americas Current ZIKV activity ZIKV transmission • ZIKV is primarily transmitted by Aedes spp. Mosquitoes. • Infectious ZIKV particles have been demonstrated in urine, saliva, semen, blood products, and breast milk possible vehicles of transmission. • Male-to-female and male-to-male transmission of ZIKV have been reported, but only from individuals with clinical signs/symptoms of ZIKV infection. • Establishing person-to-person transmission is difficult, as contacts frequently have the same environmental exposures. -

Antibody-Mediated Enhancement Aggravates Chikungunya Virus
www.nature.com/scientificreports OPEN Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity Received: 14 July 2017 Fok-Moon Lum 1,2, Thérèse Couderc3,4, Bing-Shao Chia1,8, Ruo-Yan Ong1,9, Zhisheng Her1,10, Accepted: 17 January 2018 Angela Chow5, Yee-Sin Leo5, Yiu-Wing Kam1, Laurent Rénia1, Marc Lecuit 3,4,6 & Published: xx xx xxxx Lisa F. P. Ng1,2,7 The arthropod-transmitted chikungunya virus (CHIKV) causes a fu-like disease that is characterized by incapacitating arthralgia. The re-emergence of CHIKV and the continual risk of new epidemics have reignited research in CHIKV pathogenesis. Virus-specifc antibodies have been shown to control virus clearance, but antibodies present at sub-neutralizing concentrations can also augment virus infection that exacerbates disease severity. To explore this occurrence, CHIKV infection was investigated in the presence of CHIKV-specifc antibodies in both primary human cells and a murine macrophage cell line, RAW264.7. Enhanced attachment of CHIKV to the primary human monocytes and B cells was observed while increased viral replication was detected in RAW264.7 cells. Blocking of specifc Fc receptors (FcγRs) led to the abrogation of these observations. Furthermore, experimental infection in adult mice showed that animals had higher viral RNA loads and endured more severe joint infammation in the presence of sub-neutralizing concentrations of CHIKV-specifc antibodies. In addition, CHIKV infection in 11 days old mice under enhancing condition resulted in higher muscles viral RNA load detected and death. These observations provide the frst evidence of antibody-mediated enhancement in CHIKV infection and pathogenesis and could also be relevant for other important arboviruses such as Zika virus. -

Division of Disease Control Pump Handle
"I had an interview with the Board of Guardians of St. James's parish, on the evening of Thursday, 7th September, and represented the above circumstances to them. In consequence of what I said, the handle of the pump was removed on the following day." John Snow, 1855 April 2016 Topics Rabies Update – Laura Cronquist Disease Control Is Amassing a Small Army of Students – Tracy Miller Zika Virus Update – Laura Cronquist New Disease Control Employee! Rabies Update As of May 16, 2016, nine animals have tested positive for rabies in North Dakota, including five skunks, three cows, and one cat. Six animals tested positive for rabies in 2015, but over the previous five years, an average of 31 animals per year tested positive for rabies. North Dakota Department of Health (NDDoH) surveillance data from the past 20 years shows that skunks make up the majority of animal rabies cases in the state. While all species of mammals are susceptible to rabies virus infection, over 90% of all animal rabies cases reported to the Centers for Disease Control and Prevention (CDC) occur in wild animals. Skunks, bats, raccoons, and foxes are the animals that most often get rabies in the United States. Skunks and raccoons are particularly important as reservoirs for the rabies virus, which is the rationale behind a state law prohibiting North Dakotans from keeping a skunk or raccoon in captivity. One of the best ways to protect yourself and others from rabies is by making sure that your pets are vaccinated. Contact your veterinarian to find out whether your pets are up-to-date on their rabies vaccinations. -

Dengue Fever, Chikungunya and the Zika Virus
#57 Focus Dengue Fever, Chikungunya and the Zika Virus Arboviruses are a group of virus that can be southern regions of mainland France and transmitted between animals and humans, on the island of Réunion, Aedes albopictus and they are common to humans and many provides the sole vector for transmission. vertebrates (mammals, birds, reptiles, Transmission amphibians). There are over 500 species of Dengue Fever, Chikungunya and the Zika arbovirus, sub-divided into approximately virus are all transmitted in the same way. 10 different families, including Togaviridae, Human to human transmission takes place Flaviviridae, Reoviridae, Rhabdoviridae, International and Bunyaviridae. These viruses have RNA by mosquito vector in urban areas during with a very heterogeneous structure and are epidemics: the mosquito picks up the virus transmitted via bites from hematophagous when it bites a carrier, and then transmits it arthropods such as mosquitoes, sandflies, to a healthy person with another bite. The ticks and mites (arbovirus is short for mosquito bites people outside their homes arthropod-borne virus). throughout the day, with peak activity at dawn and dusk. The mosquitoes live in Chikungunya urban areas and lay their eggs in pools of stagnant water (250 eggs every 2 days), This disease was first described in Tanzania where they develop into larvae. The eggs in 1952. It is caused by an arbovirus of the are resistant to the cold in winter and hatch genus Alphavirus from the Togaviridae family. when weather conditions improve. It was then also described in Africa, Southeast Aedes albopictus is spreading globally; it Asia, the Indian subcontinent and the Indian has adapted to both tropical and temperate Ocean. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -

Summer Safety Guide C L I N T O N C O U N T Y H E a L T H D E P a R T M E N T
SUMMER SAFETY GUIDE C L I N T O N C O U N T Y H E A L T H D E P A R T M E N T S U M M E R 2 0 1 7 WHAT'S INSIDE... 2 7 W H A T ' S B I T I N G Y O U ? P R O T E C T Y O U R H O M E 3 8-9 M O S Q U I T O E S A N I M A L S A N D R A B I E S 4-5 10 T I C K S A N D L Y M E D I S E A S E B E D B U G S 6 11-12 P R O T E C T Y O U R S E L F S U N A N D W A T E R S A F E T Y WHAT'S BITING YOU? P R E V E N T I O N I S Y O U R B E S T D E F E N S E SUMMER HAS ARRIVED! THAT Mosquitoes West Nile virus (WNV) and Eastern MEANS SUN AND FUN, BUT IT equine encephalitis (EEE) are the most common diseases transmitted IS ALSO THE TIME OF YEAR Animals by local mosquitoes. There are no Wildlife is part of the beauty of our WHEN PEOPLE ARE MOST human vaccines for these diseases, Adirondack region, but animals are but there are simple steps you can best viewed from afar. -
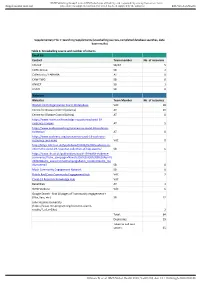
Supplementary File 1: Searching Supplements (Snowballing Sources, Completed Database Searches, Data Base Results)
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Global Health Supplementary File 1: Searching Supplements (snowballing sources, completed database searches, data base results) Table 1: Snowballing source and number of returns Email list Contact Team member No. of resources CH-CoP SB/AT 5 CORE Group SB 2 Collectivity / FARAFRA AT 0 CHW-TWG SB 0 UNICEF SB 1 USAID SB 0 Websites Websites Team Member No. of resources World Health Organization Covid-19 database VdC 18 Centre for Disease Control (Atlanta) AT 10 Centre for Disease Control (Africa) AT 0 https://www.nccmt.ca/knowledge-repositories/covid-19- evidence-reviews AT 5 https://www.evidenceaid.org/coronavirus-covid-19-evidence- collection/ AT 0 https://www.cochrane.org/coronavirus-covid-19-cochrane- resources-and-news VdC 0 http://blogs.lshtm.ac.uk/hppdebated/2020/04/08/evidence-to- inform-the-covid-19-response-collection-of-hpp-papers/ SB 6 https://www.ids.ac.uk/publications/covid-19-health-evidence- summaries/?utm_campaign=News%20at%20IDS%208%20April% 202020&utm_source=emailCampaign&utm_content=&utm_me dium=email SB 0 Mesh Community Engagement Network SB 0 British Red Cross Community Engagement Hub VdC 2 Covid-19 Research Knowledge Hub VdC ReliefWeb AT 3 WHO Website VdC 6 Google Search - first 10 pages of "community engagement + (Zika, Sars, etc) SB 12 John Hopkins University (https://www.mcsprogram.org/resource-search- results/?_sf_s=Zika) 2 Total: 64 Duplicates: 29 Taken to Full text screen: 35 Gilmore B, et al.