August 2019 Vol 25, No 8, August 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Overview of Zika
Overview of Zika Alan D.T. Barrett Department of Pathology Sealy Center for Vaccine Development University of Texas Medical Branch Galveston TX Disclaimer • Over 700 papers in pubmed on Zika in the last 12 months. • Impossible to stay up to date as field is moving so fast. • Only including material in public domain. ZIKV: 1947-2006 • Zika virus (ZIKV) causes Zika fever (ZF), an acute febrile illness characterized by a rash, conjunctival injection, arthralgia, myalgia and headache. • The disease appears in all age groups with an incubation period on the order of 3-14 days and a symptomatic phase lasting about 2-7 days. • Treatment is largely symptomatic. • The illness is mild in nature with a very low rate of hospitalization. • The vast majority of patients make a full recovery and while death is rarely reported, it has primarily occurred in the immunocompromised or those with other complicating medical conditions. • Only 14 clinical cases in the literature from 1951-2006. Map showing the known distribution of Zika virus based on serosurveys, virus detection, and laboratory-diagnosed cases. Blue arrows show recent patterns of spread deduced from phylogenetic studies Weaver et al Antiviral Research, Volume 130, 2016, 69–80 ZIKV in the Americas Current ZIKV activity ZIKV transmission • ZIKV is primarily transmitted by Aedes spp. Mosquitoes. • Infectious ZIKV particles have been demonstrated in urine, saliva, semen, blood products, and breast milk possible vehicles of transmission. • Male-to-female and male-to-male transmission of ZIKV have been reported, but only from individuals with clinical signs/symptoms of ZIKV infection. • Establishing person-to-person transmission is difficult, as contacts frequently have the same environmental exposures. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -
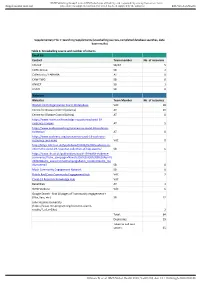
Supplementary File 1: Searching Supplements (Snowballing Sources, Completed Database Searches, Data Base Results)
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Global Health Supplementary File 1: Searching Supplements (snowballing sources, completed database searches, data base results) Table 1: Snowballing source and number of returns Email list Contact Team member No. of resources CH-CoP SB/AT 5 CORE Group SB 2 Collectivity / FARAFRA AT 0 CHW-TWG SB 0 UNICEF SB 1 USAID SB 0 Websites Websites Team Member No. of resources World Health Organization Covid-19 database VdC 18 Centre for Disease Control (Atlanta) AT 10 Centre for Disease Control (Africa) AT 0 https://www.nccmt.ca/knowledge-repositories/covid-19- evidence-reviews AT 5 https://www.evidenceaid.org/coronavirus-covid-19-evidence- collection/ AT 0 https://www.cochrane.org/coronavirus-covid-19-cochrane- resources-and-news VdC 0 http://blogs.lshtm.ac.uk/hppdebated/2020/04/08/evidence-to- inform-the-covid-19-response-collection-of-hpp-papers/ SB 6 https://www.ids.ac.uk/publications/covid-19-health-evidence- summaries/?utm_campaign=News%20at%20IDS%208%20April% 202020&utm_source=emailCampaign&utm_content=&utm_me dium=email SB 0 Mesh Community Engagement Network SB 0 British Red Cross Community Engagement Hub VdC 2 Covid-19 Research Knowledge Hub VdC ReliefWeb AT 3 WHO Website VdC 6 Google Search - first 10 pages of "community engagement + (Zika, Sars, etc) SB 12 John Hopkins University (https://www.mcsprogram.org/resource-search- results/?_sf_s=Zika) 2 Total: 64 Duplicates: 29 Taken to Full text screen: 35 Gilmore B, et al. -

(Sciurus Carolinensis) with the Contraceptive Agent Diazacontm
Integrative Zoology 2011; 6: 409-419 doi: 10.1111/j.1749-4877.2011.00247.x 1 1 2 2 3 Feeding of grey squirrels (Sciurus carolinensis) with the contraceptive 3 4 4 5 agent DiazaConTM: effect on cholesterol, hematology, and blood 5 6 6 7 chemistry 7 8 8 9 9 10 10 1 2 1 3 11 Christi A. YODER, Brenda A. MAYLE, Carol A. FURCOLOW, David P. COWAN and 11 12 Kathleen A. FAGERSTONE1 12 13 13 1 2 14 National Wildlife Research Center, Fort Collins, Colorado, USA, Forest Research, Alice Holt Lodge, Farnham, Surrey, UK and 14 15 3Central Science Laboratory, Sand Hutton, York, UK 15 16 16 17 17 18 18 19 Abstract 19 20 Grey squirrels (Sciurus carolinensis) are an invasive species in Britain and Italy. They have replaced native 20 21 red squirrels (Sciurus vulgaris) throughout most of Britain, and cause damage to trees. Currently, lethal con- 21 22 trol is used to manage grey squirrel populations in Britain, but nonlethal methods might be more acceptable to 22 23 the public. One such method is contraception with 20,25-diazacholesterol dihydrochloride (DiazaConTM). Di- 23 24 azaConTM inhibits the conversion of desmosterol to cholesterol, resulting in increasing desmosterol concentra- 24 25 tions and decreasing cholesterol concentrations. Because cholesterol is needed for the synthesis of steroid repro- 25 26 ductive hormones, such as progesterone and testosterone, inhibition of cholesterol synthesis indirectly inhibits 26 27 reproduction. Desmosterol is used as a marker of efficacy in laboratory studies with species that do not repro- 27 28 duce readily in captivity. -

EDITORIAL Zika Virus
EDITORIAL Bangladesh Medical Journal Khulna Zika virus - A global concern Maternal infection with Zika virus during the first trimester of pregnancy increases the risk of microcephaly in the baby. In late 2015, there were reports of a dramatic increase in the prevalence Vol. 49 No. 1 & 2 June & December 2016 of microcephaly in Brazil, coinciding with an outbreak of the Zika virus several months earlier. The WHO declared a Public The Journal is published twice a year in the Health Emergency of International Concern on 28 January 2016, months of June and December keeping in view the lesson learnt from the African Ebola outbreak.1 On March 22, 2016, WHO Director-General briefs the media that the status of Zika has changed from a mild medical curiosity to a disease with severe public health implications in less than a year. Subsequently, a causal association was acknowledged by WHO and Centers for Disease Control and Prevention (CDC) in April, 2016. With the steady increase in microcephaly, the Brazilian Ministry of Health set up a surveillance system and upto June 4, 2016, 7830 suspected cases 2 had been reported. Editorial Board Epidemiology: Zika virus (ZiV) continues to spread geographically to areas where competent vectors are present. Editor Although a decline in cases of Zika infection has been reported in Choudhury Habibur Rasul some countries, in later parts of the year, vigilance needs to remain high. Seventy-five countries and territories have reported Members evidence of mosquito-borne Zika virus transmission which are 3 SK Ballav divided in three categories. Md Mahmud Hassan The Zika virus outbreak made an unprecedented health crisis with Kazi Hafizur Rahman enormous health and social costs. -

Zika Incident Response Playbook
Zika Incident Response Playbook 2017 Zika Incident Response Playbook Zika Incident Response Playbook Table of Contents HAZARD OVERVIEW ……………………………………………………… PAGE 2 Transmission Symptoms Florida Risk Assessment INCIDENT MANAGEMENT STRUCTURE …………………………………… PAGE 14 INCIDENT OBJECTIVES ………………………………………………….. PAGE 15 Overarching Incident Priorities Phase 1: Imported Cases with Vector Present, No Local Transmission Phase 2: Confirmed Local Transmission in Florida, Single Case / Cluster Phase 3: Widespread Local Transmission within Single County Phase 4: Widespread Local Transmission in Multiple Counties ESSENTIAL ELEMENTS OF INFORMATION ……………………………….. PAGE 25 ADVANCED PLANNING CONSIDERATIONS ..……………………………... PAGE 26 SUPPORTING PLANS AND PROCEDURES ……………………………… PAGE 29 PRE-SCRIPTED MESSAGES……………………………………………… PAGE 31 Version 4.0 February, 2017 Page 1 Zika Incident Response Playbook HAZARD OVERVIEW Zika fever is a febrile illness caused by a mosquito-borne virus similar to those that cause dengue and West Nile virus infection. Since May 2015, local Zika virus transmission has been identified in throughout the Americas, including Puerto Rico, Texas and Florida. Outbreaks have previously been reported in Africa, Southeast Asia, and the Pacific Islands. Cases of Zika fever continue to be reported in travelers returning to the continental United States. Zika has been associated with fetal abnormalities, specifically microcephaly. Microcephaly is a birth defect where a baby’s head is smaller than expected when compared to babies of the same sex and age. Babies with microcephaly often have smaller brains that might not have developed properly. Adverse pregnancy and infant outcomes associated with Zika virus infection during pregnancy are being studied. Zika has also been associated with neurological complications, including Guillain-Barré syndrome (GBS). GBS is a rare disorder in which a person’s immune system damages their nerve cells, causing muscle weakness and sometimes paralysis. -

Parasite Ecology and the Conservation Biology of Black Rhinoceros (Diceros Bicornis)
Parasite Ecology and the Conservation Biology of Black Rhinoceros (Diceros bicornis) by Andrew Paul Stringer A thesis submitted to Victoria University of Wellington in fulfilment of the requirement for the degree of Doctor of Philosophy Victoria University of Wellington 2016 ii This thesis was conducted under the supervision of: Dr Wayne L. Linklater Victoria University of Wellington Wellington, New Zealand The animals used in this study were treated ethically and the protocols used were given approval from the Victoria University of Wellington Animal Ethics Committee (ref: 2010R6). iii iv Abstract This thesis combines investigations of parasite ecology and rhinoceros conservation biology to advance our understanding and management of the host-parasite relationship for the critically endangered black rhinoceros (Diceros bicornis). My central aim was to determine the key influences on parasite abundance within black rhinoceros, investigate the effects of parasitism on black rhinoceros and how they can be measured, and to provide a balanced summary of the advantages and disadvantages of interventions to control parasites within threatened host species. Two intestinal helminth parasites were the primary focus of this study; the strongyle nematodes and an Anoplocephala sp. tapeworm. The non-invasive assessment of parasite abundance within black rhinoceros is challenging due to the rhinoceros’s elusive nature and rarity. Hence, protocols for faecal egg counts (FECs) where defecation could not be observed were tested. This included testing for the impacts of time since defecation on FECs, and whether sampling location within a bolus influenced FECs. Also, the optimum sample size needed to reliably capture the variation in parasite abundance on a population level was estimated. -

Implications of Squirrelpox Virus for Successful Red Squirrel Translocations Within Mainland UK
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Bangor University Research Portal Recalibrating Risk: Implications of squirrelpox virus for successful red ANGOR UNIVERSITY squirrel translocations within mainland UK Shuttleworth, Craig; Brady, Deborah ; Cross, Paul; Gardner, Laura ; Greenwood, Andrew ; Jackson, Nick ; McKinney, Conor ; Robinson, Nikki ; Trotter, Stephen ; Valle, Simon; Wood, Kim ; Hayward, Matt Conservation Science and Practice DOI: 10.1111/csp2.321 PRIFYSGOL BANGOR / B E-pub ahead of print: 20/11/2020 Publisher's PDF, also known as Version of record Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): Shuttleworth, C., Brady, D., Cross, P., Gardner, L., Greenwood, A., Jackson, N., McKinney, C., Robinson, N., Trotter, S., Valle, S., Wood, K., & Hayward, M. (2020). Recalibrating Risk: Implications of squirrelpox virus for successful red squirrel translocations within mainland UK. Conservation Science and Practice. https://doi.org/10.1111/csp2.321 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

High Dose Intravenous Vitamin C Treatment for Zika Fever
JOM Volume 31, Number 1, 2016 Case Report 19 High Dose Intravenous Vitamin C Treatment for Zika Fever Michael J. Gonzalez, NMD, DSc, PhD, FACN;1 Miguel J. Berdie,l MD;2 Jorgé R. Miranda- Massari, PharmD;2 Jorgé Duconge, PhD;1 Joshua L. Rodríguez-López, BS;3 Pedro A. Adrover-López, BS 3 ¹University of Puerto Rico, Medical Sciences Campus, Schools of Public Health and Pharmacy, San Juan, PR, 00936-5067 ²Berdiel Clinic, Ponce, PR, 00716 3Ponce Health Sciences University, School of Medicine, Ponce, PR, 00716 Abstract !e Zika Fever is a viral disease caused by a single-stranded RNA virus from the Fla- vivirus genus, Flaviviridae family, from the Spondweni group. Its transmission occurs through mosquito vectors, principally Aedes Aegypti. !e most common symptoms of Zika are fever, rash, joint pain, and conjunctivitis (red eyes). Other common symptoms include muscle pain and headache. As of now, no vaccine exists for the virus and no o"cial treatment has been developed aside from standard procedures of the use of acetaminophen (paracetamol) and non-steroidal anti-in#ammatory drugs. !is is a case report of a 54 year-old Hispanic female who arrived at the clinic with symptomatology congruent with the Zika fever. !e patient was treated with high doses of intravenous vitamin C over three days. !e symptoms resolved after the infusions without any side e$ects at day four. Re- covery from this viral infection takes normally around two weeks. Based on the positive outcome in this case, we propose that intravenous vitamin C should be studied further as a potential treatment for acute viral infections. -

Nigeria Hit by Unprecedented Lassa Fever Outbreak Leslie Roberts
NEWS At Olorgesailie in Kenya, big hand axes (left) gave way INFECTIOUS DISEASE to smaller, more precise blades and points (right). more extreme. More than 80% of mammal Nigeria hit by unprecedented species had vanished and new kinds of el- ephants, pigs, foxes, and springboks gathered at tree-lined streams. MSA tools—relatively Lassa fever outbreak sophisticated blades and points that would have been hafted onto spears—were plentiful. As efforts to contain it mount, researchers are racing to find The site yielded no human fossils in this out what is driving this year’s surge in cases and deaths key time frame, so researchers can’t be sure who the new toolmakers were. But discover- ies elsewhere offer a strong hint. For years By Leslie Roberts Already, Nigeria’s fragile health care sys- archaeologists had thought the MSA tools tem is overwhelmed. The one dedicated were too old to have been made by our spe- y early January, it was clear some- Lassa fever ward in the country at Irrua cies. Then, last year, fossils resembling H. sa- thing “really, really extraordinary” Specialist Teaching Hospital in Edo state piens were found near MSA tools and dated was going on in Nigeria, says Lorenzo has just 24 beds. Without access to proper to nearly 300,000 years ago at Jebel Irhoud Pomarico of the Alliance for Interna- training and personal protective equip- in Morocco (Science, 9 June 2017, p. 993)— tional Medical Action (ALIMA). Cases ment, health care workers continue to timing that fits the Olorgesailie chronology. of Lassa fever, a rare viral hemor- become infected—by now 16 cases have Features of the MSA tools also suggest they Brhagic disease, were skyrocketing across the been reported, with one additional death. -

Northern News
NORTHERN RED SQUIRRELS HELPING OUR REDS STAND UP FOR THEMSELVES Issue 2 www.northernredsquirrels.co.uk Autumn 2008 NORTHERN NEWS Diagnosis of viral infections in the red squirrel (Sciurus vulgaris) by Transmission Electron Microscopy (TEM) By David Everest, VLA The Veterinary Laboratories Agency‟s (VLA) electron microscopy unit, established in 1965, undertakes analyses in biological samples from many species, with samples submitted by our regional laboratories, other institutes and private veterinary practitioners. An aspect of the work involves the confirmation of viral particles using the negative staining TEM technique. This is particularly used for the diagnoses of viral infections, both squirrel pox and adenovirus in the red squirrel. The technique involves grinding the scab or skin lesions in phosphate buffer, drying a drop of extract onto a support grid, negatively staining it with phosphotungstic acid, and subsequently observing virus particles when examined by TEM. A micrograph (photograph) is taken as a permanent record and we possess both micrograph and negative archives from every positive since 1971. In 1998, the VLA formed its Diseases of Wildlife Scheme, co-ordinated by Paul Duff at our Penrith laboratory, whereby tissue from suspect pox squirrels and other species of British wildlife could be sent for analysis alongside the Institute of Zoology (IOZ) red squirrel surveillance scheme. Excluding most cases from Formby, we have confirmed every case of squirrel pox and adenovirus in the UK, the first pox case being from Norfolk in 1981, with the next ones in 1993 from, Suffolk, submitted by the IOZ. We confirmed isolated cases in 1994, from Cumbria, Durham and from Dorset, resulting from a re-introduction trial and Lancashire and Suffolk in 1995.