Overview of Zika
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, AND ZIKA VIRUSES 7/31/2017 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, and Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology and Transmission ....................................... 6 Chikungunya Clinical Disease and Case Management ............... 7 Chikungunya Laboratory Testing .................................................. 8 Chikungunya Case Definitions ...................................................... 9 Chikungunya Case Classification Algorithm ............................... 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology and Transmission .............................................. 14 Dengue Clinical Disease and Case Management ...................... 14 Dengue Laboratory Testing ......................................................... 17 Dengue Case Definitions ............................................................ 19 Dengue Case Classification Algorithm ....................................... 23 III: ZIKA .................................................................................................................. -

Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on Suspicion of Infection During Business Hours
Dengue Fever/Severe Dengue Fever/Chikungunya Fever! Report on suspicion of infection during business hours PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background information on the disease (see Section 2), case definitions (see Section 3 for dengue and for chikungunya), and laboratory testing (see Section 4) Forward specimens to the Florida Department of Health (DOH) Bureau of Public Health Laboratories (BPHL) for confirmatory laboratory testing (as needed) Inform local mosquito control personnel of suspected chikungunya or dengue case as soon as possible (if applicable) Inform state Arbovirus Surveillance Coordinator on suspicion of locally acquired arbovirus infection Contact provider (see Section 5A) Interview case-patient Review disease facts (see Section 2) Mode of transmission Ask about exposure to relevant risk factors (see Section 5. Case Investigation) History of travel, outdoor activities, and mosquito bites two weeks prior to onset History of febrile illness or travel for household members or other close contacts in the month prior to onset History of previous arbovirus infection or vaccination (yellow fever, Japanese encephalitis) Provide education on transmission and prevention (see Section 6) Awareness of mosquito-borne diseases Drain standing water at least weekly to stop mosquitoes from multiplying Discard items that collect water and are not being used Cover skin with clothing or Environmental Protection Agency (EPA)-registered repellent such as DEET (N,N-diethyl-meta-toluamide) Use permethrin on clothing (not skin) according to manufacturer’s directions Cover doors and windows with intact screens to keep mosquitoes out of the house Enter additional data obtained from interview into Merlin (see Section 5D) Arrange for a convalescent specimen to be taken (if necessary) Dengue/Chikungunya Guide to Surveillance and Investigation Dengue Fever/Severe Dengue/Chikungunya 1. -
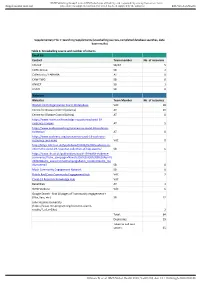
Supplementary File 1: Searching Supplements (Snowballing Sources, Completed Database Searches, Data Base Results)
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Global Health Supplementary File 1: Searching Supplements (snowballing sources, completed database searches, data base results) Table 1: Snowballing source and number of returns Email list Contact Team member No. of resources CH-CoP SB/AT 5 CORE Group SB 2 Collectivity / FARAFRA AT 0 CHW-TWG SB 0 UNICEF SB 1 USAID SB 0 Websites Websites Team Member No. of resources World Health Organization Covid-19 database VdC 18 Centre for Disease Control (Atlanta) AT 10 Centre for Disease Control (Africa) AT 0 https://www.nccmt.ca/knowledge-repositories/covid-19- evidence-reviews AT 5 https://www.evidenceaid.org/coronavirus-covid-19-evidence- collection/ AT 0 https://www.cochrane.org/coronavirus-covid-19-cochrane- resources-and-news VdC 0 http://blogs.lshtm.ac.uk/hppdebated/2020/04/08/evidence-to- inform-the-covid-19-response-collection-of-hpp-papers/ SB 6 https://www.ids.ac.uk/publications/covid-19-health-evidence- summaries/?utm_campaign=News%20at%20IDS%208%20April% 202020&utm_source=emailCampaign&utm_content=&utm_me dium=email SB 0 Mesh Community Engagement Network SB 0 British Red Cross Community Engagement Hub VdC 2 Covid-19 Research Knowledge Hub VdC ReliefWeb AT 3 WHO Website VdC 6 Google Search - first 10 pages of "community engagement + (Zika, Sars, etc) SB 12 John Hopkins University (https://www.mcsprogram.org/resource-search- results/?_sf_s=Zika) 2 Total: 64 Duplicates: 29 Taken to Full text screen: 35 Gilmore B, et al. -

EDITORIAL Zika Virus
EDITORIAL Bangladesh Medical Journal Khulna Zika virus - A global concern Maternal infection with Zika virus during the first trimester of pregnancy increases the risk of microcephaly in the baby. In late 2015, there were reports of a dramatic increase in the prevalence Vol. 49 No. 1 & 2 June & December 2016 of microcephaly in Brazil, coinciding with an outbreak of the Zika virus several months earlier. The WHO declared a Public The Journal is published twice a year in the Health Emergency of International Concern on 28 January 2016, months of June and December keeping in view the lesson learnt from the African Ebola outbreak.1 On March 22, 2016, WHO Director-General briefs the media that the status of Zika has changed from a mild medical curiosity to a disease with severe public health implications in less than a year. Subsequently, a causal association was acknowledged by WHO and Centers for Disease Control and Prevention (CDC) in April, 2016. With the steady increase in microcephaly, the Brazilian Ministry of Health set up a surveillance system and upto June 4, 2016, 7830 suspected cases 2 had been reported. Editorial Board Epidemiology: Zika virus (ZiV) continues to spread geographically to areas where competent vectors are present. Editor Although a decline in cases of Zika infection has been reported in Choudhury Habibur Rasul some countries, in later parts of the year, vigilance needs to remain high. Seventy-five countries and territories have reported Members evidence of mosquito-borne Zika virus transmission which are 3 SK Ballav divided in three categories. Md Mahmud Hassan The Zika virus outbreak made an unprecedented health crisis with Kazi Hafizur Rahman enormous health and social costs. -

Zika Incident Response Playbook
Zika Incident Response Playbook 2017 Zika Incident Response Playbook Zika Incident Response Playbook Table of Contents HAZARD OVERVIEW ……………………………………………………… PAGE 2 Transmission Symptoms Florida Risk Assessment INCIDENT MANAGEMENT STRUCTURE …………………………………… PAGE 14 INCIDENT OBJECTIVES ………………………………………………….. PAGE 15 Overarching Incident Priorities Phase 1: Imported Cases with Vector Present, No Local Transmission Phase 2: Confirmed Local Transmission in Florida, Single Case / Cluster Phase 3: Widespread Local Transmission within Single County Phase 4: Widespread Local Transmission in Multiple Counties ESSENTIAL ELEMENTS OF INFORMATION ……………………………….. PAGE 25 ADVANCED PLANNING CONSIDERATIONS ..……………………………... PAGE 26 SUPPORTING PLANS AND PROCEDURES ……………………………… PAGE 29 PRE-SCRIPTED MESSAGES……………………………………………… PAGE 31 Version 4.0 February, 2017 Page 1 Zika Incident Response Playbook HAZARD OVERVIEW Zika fever is a febrile illness caused by a mosquito-borne virus similar to those that cause dengue and West Nile virus infection. Since May 2015, local Zika virus transmission has been identified in throughout the Americas, including Puerto Rico, Texas and Florida. Outbreaks have previously been reported in Africa, Southeast Asia, and the Pacific Islands. Cases of Zika fever continue to be reported in travelers returning to the continental United States. Zika has been associated with fetal abnormalities, specifically microcephaly. Microcephaly is a birth defect where a baby’s head is smaller than expected when compared to babies of the same sex and age. Babies with microcephaly often have smaller brains that might not have developed properly. Adverse pregnancy and infant outcomes associated with Zika virus infection during pregnancy are being studied. Zika has also been associated with neurological complications, including Guillain-Barré syndrome (GBS). GBS is a rare disorder in which a person’s immune system damages their nerve cells, causing muscle weakness and sometimes paralysis. -

High Dose Intravenous Vitamin C Treatment for Zika Fever
JOM Volume 31, Number 1, 2016 Case Report 19 High Dose Intravenous Vitamin C Treatment for Zika Fever Michael J. Gonzalez, NMD, DSc, PhD, FACN;1 Miguel J. Berdie,l MD;2 Jorgé R. Miranda- Massari, PharmD;2 Jorgé Duconge, PhD;1 Joshua L. Rodríguez-López, BS;3 Pedro A. Adrover-López, BS 3 ¹University of Puerto Rico, Medical Sciences Campus, Schools of Public Health and Pharmacy, San Juan, PR, 00936-5067 ²Berdiel Clinic, Ponce, PR, 00716 3Ponce Health Sciences University, School of Medicine, Ponce, PR, 00716 Abstract !e Zika Fever is a viral disease caused by a single-stranded RNA virus from the Fla- vivirus genus, Flaviviridae family, from the Spondweni group. Its transmission occurs through mosquito vectors, principally Aedes Aegypti. !e most common symptoms of Zika are fever, rash, joint pain, and conjunctivitis (red eyes). Other common symptoms include muscle pain and headache. As of now, no vaccine exists for the virus and no o"cial treatment has been developed aside from standard procedures of the use of acetaminophen (paracetamol) and non-steroidal anti-in#ammatory drugs. !is is a case report of a 54 year-old Hispanic female who arrived at the clinic with symptomatology congruent with the Zika fever. !e patient was treated with high doses of intravenous vitamin C over three days. !e symptoms resolved after the infusions without any side e$ects at day four. Re- covery from this viral infection takes normally around two weeks. Based on the positive outcome in this case, we propose that intravenous vitamin C should be studied further as a potential treatment for acute viral infections. -

August 2019 Vol 25, No 8, August 2019
® August 2019 Pregnancy and Maternal Health Pregnancy Vol 25, No 8, August 2019 EMERGING INFECTIOUS DISEASES Pages 1445–1624 DEPARTMENT OF HEALTH & HUMAN SERVICES MEDIA MAIL Public Health Service POSTAGE & FEES PAID Centers for Disease Control and Prevention (CDC) Mailstop D61, Atlanta, GA 30329-4027 PHS/CDC Official Business Permit No. G 284 Penalty for Private Use $300 Return Service Requested Gift of George N. and Helen M. Richard, 1964. Image © The Metropolitan Museum of Art. Image source: Art Resource, NY Resource, Art source: Image Art. of Museum Metropolitan The © Image 1964. Richard, M. Helen and N. George of Gift . Oil on canvas; 28 1/2 in x 35 7/8 in/72.4 cm x 91.1 cm. cm. 91.1 x cm in/72.4 7/8 35 x in 1/2 28 canvas; on Oil . (1890) First Steps, after Millet after Steps, First Vincent van Gogh (1853–1890). (1853–1890). Gogh van Vincent ISSN 1080-6040 Peer-Reviewed Journal Tracking and Analyzing Disease Trends Pages 1445–1624 EDITOR-IN-CHIEF D. Peter Drotman ASSOCIATE EDITORS EDITORIAL BOARD Paul M. Arguin, Atlanta, Georgia, USA Barry J. Beaty, Fort Collins, Colorado, USA Charles Ben Beard, Fort Collins, Colorado, USA Martin J. Blaser, New York, New York, USA Ermias Belay, Atlanta, Georgia, USA Christopher Braden, Atlanta, Georgia, USA David M. Bell, Atlanta, Georgia, USA Arturo Casadevall, New York, New York, USA Sharon Bloom, Atlanta, Georgia, USA Kenneth G. Castro, Atlanta, Georgia, USA Richard Bradbury, Atlanta, Georgia, USA Vincent Deubel, Shanghai, China Mary Brandt, Atlanta, Georgia, USA Christian Drosten, Charité Berlin, Germany Corrie Brown, Athens, Georgia, USA Isaac Chun-Hai Fung, Statesboro, Georgia, USA Charles H. -

Communicable Diseases Communiqué JANUARY 2016, Vol
Communicable Diseases Communiqué JANUARY 2016, Vol. 15(1) CONTENTS 1 ZOONOTIC AND VECTOR–BORNE DISEASES Page a Rabies update 2 2 SEASONAL DISEASES a Malaria update, and insecticide resistance in northern KwaZulu-Natal Province 3 b An outbreak of a vesicular rash at the National School Sport Championships, 4 3 TUBERCULOSIS AND HIV Implementation of a National HIV rapid testing quality assurance and quality im- a 5 provement programme 4 ENTERIC DISEASES a Sporadic cases of typhoid in South Africa over January 2016 6 Investigation of food and water-borne outbreaks and contact details of important b 7 resources INTERNATIONAL OUTBREAKS OF IMPORTANCE TO SOUTH AFRICAN TRAVELLERS AND 5 HEALTHCARE WORKERS a Ebola virus disease (EVD) outbreak: update 8 b Zika virus 9 c Yellow fever 11 6 MEASLES AND RUBELLA a A change in the measles vaccination schedule 11 b A cluster of rubella cases in Limpopo Province 13 7 SURVEILLANCE FOR ANTIMICROBIAL RESISTANCE a Update on carbapenemase-producing Enterobacteriaceae 13 b Global spread of antimicrobial resistance to colistin 15 8 BEYOND OUR BORDERS 15 1 Communicable Diseases Communiqué JANUARY 2016, Vol. 15(1) 1 ZOONOTIC AND VECTOR-BORNE DISEASES a Rabies update A total of eight laboratory-confirmed cases of the public; 2) incorrect risk assessments by health human rabies was reported in South Africa during care workers (HCW), who may not administer PEP 2015. The most affected provinces were Limpopo or who prescribe PEP incorrectly (this may happen and the Eastern Cape (EC) with three reported if the injury is a seemingly minor scratch, or small cases each. -

Arizona Arboviral Handbook for Chikungunya, Dengue, & Zika Viruses
ARIZONA ARBOVIRAL HANDBOOK FOR CHIKUNGUNYA, DENGUE, & ZIKA VIRUSES 3/25/2016 Arizona Department of Health Services | P a g e 1 Arizona Arboviral Handbook for Chikungunya, Dengue, & Zika Viruses Arizona Arboviral Handbook for Chikungunya, Dengue, & Zika Viruses OBJECTIVES .............................................................................................................. 4 I: CHIKUNGUNYA ..................................................................................................... 5 Chikungunya Ecology & Transmission .......................................... 6 Chikungunya Clinical Disease & Case Management ................... 7 Chikungunya Laboratory Testing ................................................. 8 Chikungunya Case Definitions ..................................................... 9 Chikungunya Case Classification Algorithm .............................. 11 II: DENGUE .............................................................................................................. 12 Dengue Ecology & Transmission ................................................ 14 Dengue Clinical Disease & Case Managemen t ......................... 14 Dengue Laboratory Testing ....................................................... 17 Dengue Case Definitions ........................................................... 19 Dengue Case Classification Algorithm ...................................... 23 III: ZIKA ................................................................................................................... 24 Zika -

Zika Virus Infection 7 May 2015
Epidemiological Alert Zika virus infection 7 May 2015 The Pan American Health Organization (PAHO) / World Health Organization (WHO) recommends its Member States establish and maintain the capacity for Zika virus infection detection, clinical management and an effective public communication strategy to reduce the presence of the mosquito that transmits this disease, particularly in areas where the vector is present. Situation summary Zika virus infection The Zika virus was first isolated in 1947 in Zika Forest (Uganda), in a Rhesus monkey during a study of the This is a disease caused by the Zika virus transmission of wild yellow fever. It was first isolated in (ZIKAV), an arbovirus the flavivirus genus humans in 1952 (Uganda, Tanzania).1,2 In 1968 the virus (family Flaviviridae), very close phylogenetically to viruses such as was detected in human samples in Nigeria. 3,4 dengue, yellow fever, Japanese encephalitis, or West Nile virus. In 2007 the first major outbreak of Zika virus fever occurred on the island of Yap (Micronesia) where 185 The Zika virus is transmitted by mosquitoes suspected cases were reported, of which 49 were of the genus Aedes, in urban areas (A. confirmed and 59 were considered probable. The aegypti) as well as in the wild. outbreak lasted 13 weeks (April to July). The probable After an infected mosquito bite, the vector was identified as being Aedes hensilii, however disease symptoms usually appear the presence of the virus in the mosquito could not be following an incubation period of three determined. to twelve days. The infection may present itself as Subsequently an outbreak in French Polynesia, which asymptomatic or with a moderate began at the end of October 2013. -

Zika Virus- Emergence, Evolution, Pathology, Diagnosis, and Control: Current Global Scenario and Future Perspectives- a Comprehensive Review Raj K
Old Dominion University ODU Digital Commons Bioelectrics Publications Frank Reidy Research Center for Bioelectrics 2016 Zika Virus- Emergence, Evolution, Pathology, Diagnosis, and Control: Current Global Scenario and Future Perspectives- A Comprehensive Review Raj K. Singh Kuldeep Dhama Yashpal S. Malik Muthannan A. Ramakrishnan Kumaragurubaran Karthik See next page for additional authors Follow this and additional works at: https://digitalcommons.odu.edu/bioelectrics_pubs Part of the Public Health Commons, Veterinary Medicine Commons, and the Viruses Commons Repository Citation Singh, Raj K.; Dhama, Kuldeep; Malik, Yashpal S.; Ramakrishnan, Muthannan A.; Karthik, Kumaragurubaran; and Joshi, Sunil K., "Zika Virus- Emergence, Evolution, Pathology, Diagnosis, and Control: Current Global Scenario and Future Perspectives- A Comprehensive Review" (2016). Bioelectrics Publications. 168. https://digitalcommons.odu.edu/bioelectrics_pubs/168 Original Publication Citation Singh, R. K., Dhama, K., Malik, Y. S., Ramakrishnan, M. A., Karthik, K., Tiwari, R., . Joshi, S. K. (2016). Zika virus - emergence, evolution, pathology, diagnosis, and control: current global scenario and future perspectives - a comprehensive review. Veterinary Quarterly, 36(3), 150-175. doi:10.1080/01652176.2016.1188333 This Article is brought to you for free and open access by the Frank Reidy Research Center for Bioelectrics at ODU Digital Commons. It has been accepted for inclusion in Bioelectrics Publications by an authorized administrator of ODU Digital Commons. For more -

Florida Arbovirus Surveillance Week 15: April 11-17, 2021
Florida Arbovirus Surveillance Week 15: April 11-17, 2021 Arbovirus surveillance in Florida includes endemic mosquito-borne viruses such as West Nile virus (WNV), Eastern equine encephalitis virus (EEEV), and St. Louis encephalitis virus (SLEV), as well as exotic viruses such as dengue virus (DENV), chikungunya virus (CHIKV), Zika virus (ZIKV), and California encephalitis group viruses (CEV). Malaria, a parasitic mosquito-borne disease is also included. During the period of April 11-17, 2021, the following arboviral activity was recorded in Florida. WNV activity: No human cases of WNV infection were reported this week. No horses with WNV infection were reported this week. No sentinel chickens tested positive for antibodies to WNV this week. In 2021, positive samples from two sentinel chickens have been reported from two counties. SLEV activity: No human cases of SLEV infection were reported this week. No sentinel chickens tested positive for antibodies to SLEV this week. In 2021, no positive samples have been reported. EEEV activity: No human cases of EEEV infection were reported this week. No horses with EEEV infection were reported this week. Two sentinel chickens tested positive for antibodies to EEEV this week in Indian River County. In 2021, positive samples from one horse and 19 sentinel chickens have been reported from seven counties. International Travel-Associated Dengue Fever Cases: No cases of dengue fever were reported this week in persons that had international travel. In 2021, two travel-associated dengue fever case have been reported. Dengue Fever Cases Acquired in Florida: No cases of locally acquired dengue fever were reported this week.