Directory of Life-Limiting Conditions Hain and Devins, Cardiff, 2011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
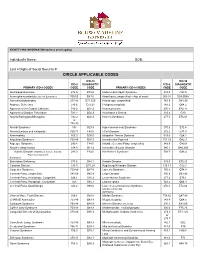
Circle Applicable Codes
IDENTIFYING INFORMATION (please print legibly) Individual’s Name: DOB: Last 4 Digits of Social Security #: CIRCLE APPLICABLE CODES ICD-10 ICD-10 ICD-9 DIAGNOSTIC ICD-9 DIAGNOSTIC PRIMARY ICD-9 CODES CODE CODE PRIMARY ICD-9 CODES CODE CODE Abetalipoproteinemia 272.5 E78.6 Hallervorden-Spatz Syndrome 333.0 G23.0 Acrocephalosyndactyly (Apert’s Syndrome) 755.55 Q87.0 Head Injury, unspecified – Age of onset: 959.01 S09.90XA Adrenaleukodystrophy 277.86 E71.529 Hemiplegia, unspecified 342.9 G81.90 Arginase Deficiency 270.6 E72.21 Holoprosencephaly 742.2 Q04.2 Agenesis of the Corpus Callosum 742.2 Q04.3 Homocystinuria 270.4 E72.11 Agenesis of Septum Pellucidum 742.2 Q04.3 Huntington’s Chorea 333.4 G10 Argyria/Pachygyria/Microgyria 742.2 Q04.3 Hurler’s Syndrome 277.5 E76.01 or 758.33 Aicardi Syndrome 333 G23.8 Hyperammonemia Syndrome 270.6 E72.4 Alcohol Embryo and Fetopathy 760.71 F84.5 I-Cell Disease 272.2 E77.0 Anencephaly 655.0 Q00.0 Idiopathic Torsion Dystonia 333.6 G24.1 Angelman Syndrome 759.89 Q93.5 Incontinentia Pigmenti 757.33 Q82.3 Asperger Syndrome 299.8 F84.5 Infantile Cerebral Palsy, unspecified 343.9 G80.9 Ataxia-Telangiectasia 334.8 G11.3 Intractable Seizure Disorder 345.1 G40.309 Autistic Disorder (Childhood Autism, Infantile 299.0 F84.0 Klinefelter’s Syndrome 758.7 Q98.4 Psychosis, Kanner’s Syndrome) Biotinidase Deficiency 277.6 D84.1 Krabbe Disease 333.0 E75.23 Canavan Disease 330.0 E75.29 Kugelberg-Welander Disease 335.11 G12.1 Carpenter Syndrome 759.89 Q87.0 Larsen’s Syndrome 755.8 Q74.8 Cerebral Palsy, unspecified 343.69 G80.9 -

2018 Etiologies by Frequencies
2018 Etiologies in Order of Frequency by Category Hereditary Syndromes and Disorders Count CHARGE Syndrome 958 Down syndrome (Trisomy 21 syndrome) 308 Usher I syndrome 252 Stickler syndrome 130 Dandy Walker syndrome 119 Cornelia de Lange 102 Goldenhar syndrome 98 Usher II syndrome 83 Wolf-Hirschhorn syndrome (Trisomy 4p) 68 Trisomy 13 (Trisomy 13-15, Patau syndrome) 60 Pierre-Robin syndrome 57 Moebius syndrome 55 Trisomy 18 (Edwards syndrome) 52 Norrie disease 38 Leber congenital amaurosis 35 Chromosome 18, Ring 18 31 Aicardi syndrome 29 Alstrom syndrome 27 Pfieffer syndrome 27 Treacher Collins syndrome 27 Waardenburg syndrome 27 Marshall syndrome 25 Refsum syndrome 21 Cri du chat syndrome (Chromosome 5p- synd) 16 Bardet-Biedl syndrome (Laurence Moon-Biedl) 15 Hurler syndrome (MPS I-H) 15 Crouzon syndrome (Craniofacial Dysotosis) 13 NF1 - Neurofibromatosis (von Recklinghausen dis) 13 Kniest Dysplasia 12 Turner syndrome 11 Usher III syndrome 10 Cockayne syndrome 9 Apert syndrome/Acrocephalosyndactyly, Type 1 8 Leigh Disease 8 Alport syndrome 6 Monosomy 10p 6 NF2 - Bilateral Acoustic Neurofibromatosis 6 Batten disease 5 Kearns-Sayre syndrome 5 Klippel-Feil sequence 5 Hereditary Syndromes and Disorders Count Prader-Willi 5 Sturge-Weber syndrome 5 Marfan syndrome 3 Hand-Schuller-Christian (Histiocytosis X) 2 Hunter Syndrome (MPS II) 2 Maroteaux-Lamy syndrome (MPS VI) 2 Morquio syndrome (MPS IV-B) 2 Optico-Cochleo-Dentate Degeneration 2 Smith-Lemli-Opitz (SLO) syndrome 2 Wildervanck syndrome 2 Herpes-Zoster (or Hunt) 1 Vogt-Koyanagi-Harada -

Level Estimates of Maternal Smoking and Nicotine Replacement Therapy During Pregnancy
Using primary care data to assess population- level estimates of maternal smoking and nicotine replacement therapy during pregnancy Nafeesa Nooruddin Dhalwani BSc MSc Thesis submitted to the University of Nottingham for the degree of Doctor of Philosophy November 2014 ABSTRACT Background: Smoking in pregnancy is the most significant preventable cause of poor health outcomes for women and their babies and, therefore, is a major public health concern. In the UK there is a wide range of interventions and support for pregnant women who want to quit. One of these is nicotine replacement therapy (NRT) which has been widely available for retail purchase and prescribing to pregnant women since 2005. However, measures of NRT prescribing in pregnant women are scarce. These measures are vital to assess its usefulness in smoking cessation during pregnancy at a population level. Furthermore, evidence of NRT safety in pregnancy for the mother and child’s health so far is nebulous, with existing studies being small or using retrospectively reported exposures. Aims and Objectives: The main aim of this work was to assess population- level estimates of maternal smoking and NRT prescribing in pregnancy and the safety of NRT for both the mother and the child in the UK. Currently, the only population-level data on UK maternal smoking are from repeated cross-sectional surveys or routinely collected maternity data during pregnancy or at delivery. These obtain information at one point in time, and there are no population-level data on NRT use available. As a novel approach, therefore, this thesis used the routinely collected primary care data that are currently available for approximately 6% of the UK population and provide longitudinal/prospectively recorded information throughout pregnancy. -

Diseases of the Digestive System (KOO-K93)
CHAPTER XI Diseases of the digestive system (KOO-K93) Diseases of oral cavity, salivary glands and jaws (KOO-K14) lijell Diseases of pulp and periapical tissues 1m Dentofacial anomalies [including malocclusion] Excludes: hemifacial atrophy or hypertrophy (Q67.4) K07 .0 Major anomalies of jaw size Hyperplasia, hypoplasia: • mandibular • maxillary Macrognathism (mandibular)(maxillary) Micrognathism (mandibular)( maxillary) Excludes: acromegaly (E22.0) Robin's syndrome (087.07) K07 .1 Anomalies of jaw-cranial base relationship Asymmetry of jaw Prognathism (mandibular)( maxillary) Retrognathism (mandibular)(maxillary) K07.2 Anomalies of dental arch relationship Cross bite (anterior)(posterior) Dis to-occlusion Mesio-occlusion Midline deviation of dental arch Openbite (anterior )(posterior) Overbite (excessive): • deep • horizontal • vertical Overjet Posterior lingual occlusion of mandibular teeth 289 ICO-N A K07.3 Anomalies of tooth position Crowding Diastema Displacement of tooth or teeth Rotation Spacing, abnormal Transposition Impacted or embedded teeth with abnormal position of such teeth or adjacent teeth K07.4 Malocclusion, unspecified K07.5 Dentofacial functional abnormalities Abnormal jaw closure Malocclusion due to: • abnormal swallowing • mouth breathing • tongue, lip or finger habits K07.6 Temporomandibular joint disorders Costen's complex or syndrome Derangement of temporomandibular joint Snapping jaw Temporomandibular joint-pain-dysfunction syndrome Excludes: current temporomandibular joint: • dislocation (S03.0) • strain (S03.4) K07.8 Other dentofacial anomalies K07.9 Dentofacial anomaly, unspecified 1m Stomatitis and related lesions K12.0 Recurrent oral aphthae Aphthous stomatitis (major)(minor) Bednar's aphthae Periadenitis mucosa necrotica recurrens Recurrent aphthous ulcer Stomatitis herpetiformis 290 DISEASES OF THE DIGESTIVE SYSTEM Diseases of oesophagus, stomach and duodenum (K20-K31) Ill Oesophagitis Abscess of oesophagus Oesophagitis: • NOS • chemical • peptic Use additional external cause code (Chapter XX), if desired, to identify cause. -

Zellweger Syndrome: Biochemical and Morphological Studies on Two Patients Treated with Clofibrate
003 1-3998/85/19 12-1356$02.00/0 PEDIATRIC RESEARCH Vol. 19, No. 12, 1985 Copyright O 1985 International Pediatric Research Foundation, Inc. Printed in (I.S. A. Zellweger Syndrome: Biochemical and Morphological Studies on Two Patients Treated with Clofibrate PAUL B. LAZAROW, VIRGINIA BLACK, HELEN SHIO, YUKIO FUJIKI, AMIYA K. HAJRA, NABANITA S. DATTA, BABU S. BANGARU, AND JOSEPH DANCIS The Rockefiller University, 1230 York Avenue, New York, New York 10021 [P.B.L., H.S., Y.F.] Departments of Cell Biology [V.B.] and Pediatrics [B.S.B., J.D.], New York University School of Medicine, 550 First Avenue, New York, NY 10016; and Neuroscience Laboratory, The University of Michigan, Ann Arbor, Michigan 48109 [A.K.H., N.S.D.] ABSTRACT. Two infants with Zellweger syndrome (cer- genetic change, which is implied by the observed autosomal ebro-hepato-renal syndrome) have been studied bio- recessive mode of inheritance (2)? The functions of peroxisomes chemically and morphologically. Peroxisomal enzymes in- include respiration (forming HZ02)(12), fatty acid catabolism volved in respiration, fatty acid @-oxidation,and plasmal- (13, 14), and the initial steps in plasmalogen biosynthesis (15). ogen biosynthesis were assessed. In liver, catalase was The peroxisomal fatty acid P-oxidation system acts on saturated present in normal amounts but was located in the cell and unsaturated fatty acyl-CoAs with chainlengths from 6 to 26 cytosol. Dihydroxyacetone phosphate acyltransferase ac- (14, 16, 17), the sidechain of cholesterol (18), dicarboxylic fatty tivity was less than one-tenth of normal. The amount of acids (l9), and glutaryl-CoA (20). -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

Peroxisomal Bifunctional Enzyme Deficiency
Peroxisomal bifunctional enzyme deficiency. P A Watkins, … , A B Moser, M E Beard J Clin Invest. 1989;83(3):771-777. https://doi.org/10.1172/JCI113956. Research Article Peroxisomal function was evaluated in a male infant with clinical features of neonatal adrenoleukodystrophy. Very long chain fatty acid levels were elevated in both plasma and fibroblasts, and beta-oxidation of very long chain fatty acids in cultured fibroblasts was significantly impaired. Although the level of the bile acid intermediate trihydroxycoprostanoic acid was slightly elevated in plasma, phytanic acid and L-pipecolic acid levels were normal, as was plasmalogen synthesis in cultured fibroblasts. The latter three parameters distinguish this case from classical neonatal adrenoleukodystrophy. In addition, electron microscopy and catalase subcellular distribution studies revealed that, in contrast to neonatal adrenoleukodystrophy, peroxisomes were present in the patient's tissues. Immunoblot studies of peroxisomal beta- oxidation enzymes revealed that the bifunctional enzyme (enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase) was deficient in postmortem liver samples, whereas acyl-CoA oxidase and the mature form of beta-ketothiolase were present. Density gradient centrifugation of fibroblast homogenates confirmed that intact peroxisomes were present. Immunoblots of fibroblasts peroxisomal fractions showed that they contained acyl-CoA oxidase and beta-ketothiolase, but bifunctional enzyme was not detected. Northern analysis, however, revealed that mRNA coding for the bifunctional enzyme was present in the patient's fibroblasts. These results indicate that the primary biochemical defect in this patient is a deficiency of peroxisomal bifunctional enzyme. It is of interest that the phenotype of this patient resembled neonatal adrenoleukodystrophy and would not have been […] Find the latest version: https://jci.me/113956/pdf Peroxisomal Bifunctional Enzyme Deficiency Paul A. -

Zellweger Spectrum Disorder
ZELLWEGER SPECTRUM DISORDER What is it? Zellweger Spectrum Disorder (ZSD) was recently viewed as 3 separate diseases but today is categorized as set of disorders that form a spectrum, or continuum, of 1 disease. This spectrum can range from mild Infantile Refsum Disease (IRD), to moderate Neonatal Adrenoleukodystrophy (NALD), to severe Zellweger Syndrome (ZS) ZSD is also known as peroxisome biogenesis disorders (PBDs). These disorders are caused by a loss of function in important parts of your cells called “peroxisomes,” which are responsible for breaking down fats and chemicals and getting rid of waste so that your body can function properly. This disorder can affect many parts of the body from the eyes to the liver. The various body systems and functions as described below. Nutrition Malabsorption of nutrients can lead to poor growth, feeding problems, and deficiency in fat-soluble vitamins. Hearing Varying degree of hearing loss requires the child receive a yearly evaluation . Vision Vision loss is the most common problem. Neurological Improper development of the white matter in the brain (leukodystrophy) results in nerve damage and can potentially affect their development. Damage to the nerves that send information from the brain to the rest of the body (peripheral neuropathy) can often cause numbness or weakness in the hands and/or feet. Walking abnormality is the main neurological complication in adults with ZSDs. In people with mild forms of ZSDs, nerve damage to the muscles, skin, and internal organs usually begins during adolescence. Kidney Kidney issues occur in children 4 years and older and include kidney stones, kidney cyst, and kidney failure. -

Blueprint Genetics Comprehensive Growth Disorders / Skeletal
Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel Test code: MA4301 Is a 374 gene panel that includes assessment of non-coding variants. This panel covers the majority of the genes listed in the Nosology 2015 (PMID: 26394607) and all genes in our Malformation category that cause growth retardation, short stature or skeletal dysplasia and is therefore a powerful diagnostic tool. It is ideal for patients suspected to have a syndromic or an isolated growth disorder or a skeletal dysplasia. About Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders This panel covers a broad spectrum of diseases associated with growth retardation, short stature or skeletal dysplasia. Many of these conditions have overlapping features which can make clinical diagnosis a challenge. Genetic diagnostics is therefore the most efficient way to subtype the diseases and enable individualized treatment and management decisions. Moreover, detection of causative mutations establishes the mode of inheritance in the family which is essential for informed genetic counseling. For additional information regarding the conditions tested on this panel, please refer to the National Organization for Rare Disorders and / or GeneReviews. Availability 4 weeks Gene Set Description Genes in the Comprehensive Growth Disorders / Skeletal Dysplasias and Disorders Panel and their clinical significance Gene Associated phenotypes Inheritance ClinVar HGMD ACAN# Spondyloepimetaphyseal dysplasia, aggrecan type, AD/AR 20 56 Spondyloepiphyseal dysplasia, Kimberley -

Physical Assessment of the Newborn: Part 3
Physical Assessment of the Newborn: Part 3 ® Evaluate facial symmetry and features Glabella Nasal bridge Inner canthus Outer canthus Nasal alae (or Nare) Columella Philtrum Vermillion border of lip © K. Karlsen 2013 © K. Karlsen 2013 Forceps Marks Assess for symmetry when crying . Asymmetry cranial nerve injury Extent of injury . Eye involvement ophthalmology evaluation © David A. ClarkMD © David A. ClarkMD © K. Karlsen 2013 © K. Karlsen 2013 The S.T.A.B.L.E® Program © 2013. Handout may be reproduced for educational purposes. 1 Physical Assessment of the Newborn: Part 3 Bruising Moebius Syndrome Congenital facial paralysis 7th cranial nerve (facial) commonly Face presentation involved delivery . Affects facial expression, sense of taste, salivary and lacrimal gland innervation Other cranial nerves may also be © David A. ClarkMD involved © David A. ClarkMD . 5th (trigeminal – muscles of mastication) . 6th (eye movement) . 8th (balance, movement, hearing) © K. Karlsen 2013 © K. Karlsen 2013 Position, Size, Distance Outer canthal distance Position, Size, Distance Outer canthal distance Normal eye spacing Normal eye spacing inner canthal distance = inner canthal distance = palpebral fissure length Inner canthal distance palpebral fissure length Inner canthal distance Interpupillary distance (midpoints of pupils) distance of eyes from each other Interpupillary distance Palpebral fissure length (size of eye) Palpebral fissure length (size of eye) © K. Karlsen 2013 © K. Karlsen 2013 Position, Size, Distance Outer canthal distance -

Soonerstart Automatic Qualifying Syndromes and Conditions
SoonerStart Automatic Qualifying Syndromes and Conditions - Appendix O Abetalipoproteinemia Acanthocytosis (see Abetalipoproteinemia) Accutane, Fetal Effects of (see Fetal Retinoid Syndrome) Acidemia, 2-Oxoglutaric Acidemia, Glutaric I Acidemia, Isovaleric Acidemia, Methylmalonic Acidemia, Propionic Aciduria, 3-Methylglutaconic Type II Aciduria, Argininosuccinic Acoustic-Cervico-Oculo Syndrome (see Cervico-Oculo-Acoustic Syndrome) Acrocephalopolysyndactyly Type II Acrocephalosyndactyly Type I Acrodysostosis Acrofacial Dysostosis, Nager Type Adams-Oliver Syndrome (see Limb and Scalp Defects, Adams-Oliver Type) Adrenoleukodystrophy, Neonatal (see Cerebro-Hepato-Renal Syndrome) Aglossia Congenita (see Hypoglossia-Hypodactylia) Aicardi Syndrome AIDS Infection (see Fetal Acquired Immune Deficiency Syndrome) Alaninuria (see Pyruvate Dehydrogenase Deficiency) Albers-Schonberg Disease (see Osteopetrosis, Malignant Recessive) Albinism, Ocular (includes Autosomal Recessive Type) Albinism, Oculocutaneous, Brown Type (Type IV) Albinism, Oculocutaneous, Tyrosinase Negative (Type IA) Albinism, Oculocutaneous, Tyrosinase Positive (Type II) Albinism, Oculocutaneous, Yellow Mutant (Type IB) Albinism-Black Locks-Deafness Albright Hereditary Osteodystrophy (see Parathyroid Hormone Resistance) Alexander Disease Alopecia - Mental Retardation Alpers Disease Alpha 1,4 - Glucosidase Deficiency (see Glycogenosis, Type IIA) Alpha-L-Fucosidase Deficiency (see Fucosidosis) Alport Syndrome (see Nephritis-Deafness, Hereditary Type) Amaurosis (see Blindness) Amaurosis -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008.