Activation of Thewnt–Яcatenin Pathway in a Cell Population on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Sec14-Like Phosphatidylinositol Transfer Proteins Sec14l3/SEC14L2
RESEARCH ARTICLE The Sec14-like phosphatidylinositol transfer proteins Sec14l3/SEC14L2 act as GTPase proteins to mediate Wnt/Ca2+ signaling Bo Gong, Weimin Shen, Wanghua Xiao, Yaping Meng, Anming Meng*, Shunji Jia* State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, China Abstract The non-canonical Wnt/Ca2+ signaling pathway plays important roles in embryonic development, tissue formation and diseases. However, it is unclear how the Wnt ligand-stimulated, G protein-coupled receptor Frizzled activates phospholipases for calcium release. Here, we report that the zebrafish/human phosphatidylinositol transfer protein Sec14l3/SEC14L2 act as GTPase proteins to transduce Wnt signals from Frizzled to phospholipase C (PLC). Depletion of sec14l3 attenuates Wnt/Ca2+ responsive activity and causes convergent and extension (CE) defects in zebrafish embryos. Biochemical analyses in mammalian cells indicate that Sec14l3-GDP forms complex with Frizzled and Dishevelled; Wnt ligand binding of Frizzled induces translocation of Sec14l3 to the plasma membrane; and then Sec14l3-GTP binds to and activates phospholipase Cd4a (Plcd4a); subsequently, Plcd4a initiates phosphatidylinositol-4,5-bisphosphate (PIP2) signaling, ultimately stimulating calcium release. Furthermore, Plcd4a can act as a GTPase-activating protein to accelerate the hydrolysis of Sec14l3-bound GTP to GDP. Our data provide a new insight into GTPase protein-coupled Wnt/Ca2+ signaling transduction. DOI: 10.7554/eLife.26362.001 *For correspondence: mengam@ mail.tsinghua.edu.cn (AM); jiasj@ mail.tsinghua.edu.cn (SJ) Competing interests: The Introduction authors declare that no Wnt ligands, a large family of secreted lipoglycoproteins, control a large number of developmental competing interests exist. -

Wnt7b Regulates Placental Development in Miceprovided by Elsevier - Publisher Connector
Developmental Biology 237, 324–332 (2001) doi:10.1006/dbio.2001.0373, available online at http://www.idealibrary.com on View metadata, citation and similar papers at core.ac.uk brought to you by CORE Wnt7b Regulates Placental Development in Miceprovided by Elsevier - Publisher Connector Brian A. Parr,1 Valerie A. Cornish, Myron I. Cybulsky,2 and Andrew P. McMahon3 MCD Biology, University of Colorado, 347 UCB, Boulder, Colorado 80309-0347 Secreted Wnt proteins regulate many developmental processes in multicellular organisms. We have generated a targeted mutation in the mouse Wnt7b gene. Homozygous Wnt7b mutant mice die at midgestation stages as a result of placental abnormalities. Wnt7b expression in the chorion is required for fusion of the chorion and allantois during placental development. The ␣4 integrin protein, required for chorioallantoic fusion, is not expressed by cells in the mutant chorion. Wnt7b also is required for normal organization of cells in the chorionic plate. Thus, Wnt7b signaling is central to the early stages of placental development in mammals. © 2001 Academic Press Key Words: Wnt7b; placenta; chorion; ␣4 integrin. INTRODUCTION rupted, a variety of placental defects are observed. For example, mutations in the genes for scatter factor, the EGF The mammalian placenta is a complex structure requir- receptor, or the LIF receptor produce trophoblast cell abnor- ing the coordinated growth and differentiation of maternal malities in the placenta (Threadgill et al., 1995; Uehara et and fetal tissues. The amnion, yolk sac, chorion, and al., 1995; Ware et al., 1995). allantois are the extraembryonic tissues most closely asso- A number of mouse mutants with defects in the chori- ciated with the embryo during early stages of mouse devel- onic component of the placenta have been identified. -

WNT2 and WNT7B Cooperative Signaling in Lung Development
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2012 WNT2 and WNT7B Cooperative Signaling in Lung Development Mayumi Miller University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Developmental Biology Commons, and the Molecular Biology Commons Recommended Citation Miller, Mayumi, "WNT2 and WNT7B Cooperative Signaling in Lung Development" (2012). Publicly Accessible Penn Dissertations. 674. https://repository.upenn.edu/edissertations/674 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/674 For more information, please contact [email protected]. WNT2 and WNT7B Cooperative Signaling in Lung Development Abstract The development of a complex organ, such as the lung, relies upon precisely controlled temporal and spatial expression patterns of signaling pathways for proper specification and differentiation of the cell types required to build a lung. While progress has been made in dissecting the network of signaling pathways and the integration of their positive and negative feedback mechanisms, there is still much to discover. For example, the Wnt signaling pathway is required for lung specification and growth, but a combinatorial role for Wnt ligands has not been investigated. In this dissertation, I combine mouse genetic models and in vitro and ex vivo lung culture assays, to determine a cooperative role for Wnt2 and Wnt7b in the developing lung. This body of work reveals the requirement of cooperative signaling between Wnt2 and Wnt7b for smooth muscle development and proximal to distal patterning of the lung. Additional findings er veal a role for the Pdgf pathway and homeobox genes in potentiating this cooperation. -

Wnt Proteins Synergize to Activate Β-Catenin Signaling Anshula Alok1, Zhengdeng Lei1,2,*, N
© 2017. Published by The Company of Biologists Ltd | Journal of Cell Science (2017) 130, 1532-1544 doi:10.1242/jcs.198093 RESEARCH ARTICLE Wnt proteins synergize to activate β-catenin signaling Anshula Alok1, Zhengdeng Lei1,2,*, N. Suhas Jagannathan1,2, Simran Kaur1,‡, Nathan Harmston2, Steven G. Rozen1,2, Lisa Tucker-Kellogg1,2 and David M. Virshup1,3,§ ABSTRACT promoters and enhancers to drive expression with distinct Wnt ligands are involved in diverse signaling pathways that are active developmental timing and tissue specificity. However, in both during development, maintenance of tissue homeostasis and in normal and disease states, multiple Wnt genes are often expressed various disease states. While signaling regulated by individual Wnts in combination (Akiri et al., 2009; Bafico et al., 2004; Benhaj et al., has been extensively studied, Wnts are rarely expressed alone, 2006; Suzuki et al., 2004). For example, stromal cells that support the and the consequences of Wnt gene co-expression are not well intestinal stem cell niche express at least six different Wnts at the same understood. Here, we studied the effect of co-expression of Wnts on time (Kabiri et al., 2014). While in isolated instances, specific Wnt β the β-catenin signaling pathway. While some Wnts are deemed ‘non- pairs have been shown to combine to enhance -catenin signaling canonical’ due to their limited ability to activate β-catenin when during embryonic development, whether this is a general expressed alone, unexpectedly, we find that multiple Wnt combinations phenomenon remains unclear (Cha et al., 2008; Cohen et al., 2012; can synergistically activate β-catenin signaling in multiple cell types. -

Control of Wnt5b Secretion by Wntless Modulates Chondrogenic Cell Proliferation Through Fine-Tuning Fgf3 Expression Bo-Tsung Wu1,2, Shih-Hsien Wen1,2, Sheng-Ping L
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 2328-2339 doi:10.1242/jcs.167403 RESEARCH ARTICLE Control of Wnt5b secretion by Wntless modulates chondrogenic cell proliferation through fine-tuning fgf3 expression Bo-Tsung Wu1,2, Shih-Hsien Wen1,2, Sheng-Ping L. Hwang3, Chang-Jen Huang1,2 and Yung-Shu Kuan1,2,4,* ABSTRACT activities to achieve the proper proliferation, differentiation or Wnts and Fgfs regulate various tissues development in migration responses is still relatively limited. β vertebrates. However, how regional Wnt or Fgf activities are In vertebrates, the -catenin-mediated canonical and the non- established and how they interact in any given developmental canonical Wnt signaling pathways have both been shown to be event is elusive. Here, we investigated the Wnt-mediated involved in the processes of craniofacial skeleton formation. In craniofacial cartilage development in zebrafish and found that mice, previous observations have indicated that Wnts can either fgf3 expression in the pharyngeal pouches is differentially reduced stimulate chondrogenesis by promoting survival and differentiation along the anteroposterior axis in wnt5b mutants and wntless (wls) of migrating neural crest cells (NCCs), or inhibit chondrogenesis by morphants, but its expression is normal in wnt9a and wnt11 repressing BMP2-induced chondrocyte gene expression, depending morphants. Introducing fgf3 mRNAs rescued the cartilage defects on the developmental stage and the local tissue context (Brault et al., in Wnt5b- and Wls-deficient larvae. In wls morphants, endogenous 2001; Liu et al., 2008; Reinhold et al., 2006; Yang et al., 2003). In Wls expression is not detectable but maternally deposited Wls is zebrafish, wnt4a and wnt11r have been shown to regulate the present in eggs, which might account for the lack of axis defects in formation of pharyngeal pouches, whereas wnt5b, wnt9a and wnt11 wls morphants. -

Wnt Signaling Regulates Smooth Muscle Precursor Development in the Mouse Lung Via a Tenascin C/PDGFR Pathway
Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway Ethan David Cohen, … , Peter Lloyd Jones, Edward E. Morrisey J Clin Invest. 2009;119(9):2538-2549. https://doi.org/10.1172/JCI38079. Research Article Vascular biology Paracrine signaling from lung epithelium to the surrounding mesenchyme is important for lung SMC development and function and is a contributing factor in an array of pulmonary diseases such as bronchopulmonary dysplasia, pulmonary hypertension, and asthma. Wnt7b, which is exclusively expressed in the lung epithelium, is important for lung vascular smooth muscle integrity, but the underlying mechanism by which Wnt signaling regulates lung SMC development is unclear. In this report, we have demonstrated that Wnt7b regulates a program of mesenchymal differentiation in the mouse lung that is essential for SMC development. Genetic loss-of-function studies showed that Wnt7b and β-catenin were required for expression of Pdgfrα and Pdgfrβ and proliferation in pulmonary SMC precursors. In contrast, gain-of- function studies showed that activation of Wnt signaling increased the expression of both Pdgfrα and Pdgfrβ as well as the proliferation of SMC precursors. We further showed that the effect on Pdgfr expression was, in part, mediated by direct transcriptional regulation of the ECM protein tenascin C (Tnc), which was necessary and sufficient for Pdgfrα/β expression in lung explants. Moreover, this pathway was highly upregulated in a mouse model of asthma and in lung tissue from patients with pulmonary hypertension. Together, these data define a Wnt/Tnc/Pdgfr signaling axis that is critical for smooth muscle development and disease progression in the lung. -

A Canonical to Non-Canonical Wnt Signalling Switch in Haematopoietic Stem-Cell Ageing
LETTER doi:10.1038/nature12631 A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing Maria Carolina Florian1, Kalpana J. Nattamai2, Karin Do¨rr1, Gina Marka1, Bettina U¨berle1, Virag Vas1, Christina Eckl3, Immanuel Andra¨4, Matthias Schiemann4, Robert A. J. Oostendorp3, Karin Scharffetter-Kochanek1, Hans Armin Kestler5, Yi Zheng2 & Hartmut Geiger1,2 Many organs with a high cell turnover (for example, skin, intestine LT-HSCs presented with a reduced level and primarily cytoplasmic loca- and blood) are composed of short-lived cells that require continuous lization of b-catenin (Fig. 1d, e and Supplementary Video 3). Reduced replenishment by somatic stem cells1,2. Ageing results in the inability levels of b-catenin upon ageing were specific to the LT-HSC compart- of these tissues to maintain homeostasis and it is believed that somatic ment, as more differentiated LKs (Lin2c-Kit1Sca-12 cells), LSKs stem-cell ageing is one underlying cause of tissue attrition with age (Lin2Sca-11c-Kit1 cells), lymphoid-primed multipotent progenitors or age-related diseases. Ageing of haematopoietic stem cells (HSCs) (LMPPs; Lin2c-Kit1Sca-12CD341Flk21 cells) and short-term (ST)- is associated with impaired haematopoiesis in the elderly3–6. Despite HSCs (Lin2c-Kit1Sca-12CD341Flk22 cells) (Extended Data Fig. 1h) a large amount of data describing the decline of HSC function on showed similar levels of b-catenin upon ageing (Fig. 1g and Extended ageing, the molecular mechanisms of this process remain largely Data Fig. 1i). Axin2 (an established direct downstream target of cano- unknown, which precludes rational approaches to attenuate stem- nical Wnt signalling9,15) transcript levels in aged LT-HSCs were mark- cell ageing. -

Induction of Apoptosis Increases Expression of Non-Canonical WNT Genes in Myeloid Leukemia Cell Lines
1563-1569 7/11/07 19:04 Page 1563 ONCOLOGY REPORTS 18: 1563-1569, 2007 Induction of apoptosis increases expression of non-canonical WNT genes in myeloid leukemia cell lines HAKKI OGUN SERCAN1, MELEK PEHLIVAN1, OZLENEN SIMSEK1, HALIL ATES2 and ZEYNEP SERCAN1 Depatments of 1Medical Biology and Genetics, 2Hematology/Oncology, Dokuz Eylul University Faculty of Medicine, Izmir, Turkey Received May 3, 2007; Accepted July 20, 2007 Abstract. With the aim of determining the differential and cyclin D. The presence or exclusion of co-receptors (low expression of WNT and FZD genes, before and after density lipoprotein receptor-related protein 5 and 6-LRP5/6) induction of apoptosis in BCR-ABL positive cells, we treated or secreted Wnt antagonists (soluble frizzled related proteins the myeloid cell line K562 and control cell line HL60 with - sFRPs- and Dickkopf proteins) increases the complexity of imatinib mesylate and etoposide, and analyzed relative the molecular mechanisms underlying cellular outcomes. mRNA expression levels of WNT, FZD and sFRP genes Non-canonical pathway activation is independent of ß- under normal and apoptotic conditions by real-time RT-PCR. catenin. Two non-canonical pathways have also been defined: We observed marked increase in mRNA levels of FZD4, the planar cell polarity (PCP) and the Wnt/Ca2+ pathways (3-6). FZD5, FZD7 and WNT5b, correlating with apoptotic activity There is an overlap of proposed Wnt and Fzd proteins and and independent of the agent or cell line used. Our results secondary messengers participating in these non-canonical suggest the involvement of non-canonical Wnt signaling in pathways, complicating further the interpretation of experi- executing programmed cell death in myeloid cell lines. -

The Polycomb Group Protein Ring1 Regulates Dorsoventral Patterning Of
ARTICLE https://doi.org/10.1038/s41467-020-19556-5 OPEN The Polycomb group protein Ring1 regulates dorsoventral patterning of the mouse telencephalon ✉ Hikaru Eto1,6, Yusuke Kishi 1,6 , Nayuta Yakushiji-Kaminatsui 2, Hiroki Sugishita2, Shun Utsunomiya1,3,5, ✉ Haruhiko Koseki2 & Yukiko Gotoh 1,4 1234567890():,; Dorsal-ventral patterning of the mammalian telencephalon is fundamental to the formation of distinct functional regions including the neocortex and ganglionic eminence. While Bone morphogenetic protein (BMP), Wnt, and Sonic hedgehog (Shh) signaling are known to determine regional identity along the dorsoventral axis, how the region-specific expression of these morphogens is established remains unclear. Here we show that the Polycomb group (PcG) protein Ring1 contributes to the ventralization of the mouse telencephalon. Deletion of Ring1b or both Ring1a and Ring1b in neuroepithelial cells induces ectopic expression of dorsal genes, including those for BMP and Wnt ligands, as well as attenuated expression of the gene for Shh, a key morphogen for ventralization, in the ventral telencephalon. We observe PcG protein–mediated trimethylation of histone 3 at lysine-27 and binding of Ring1B at BMP and Wnt ligand genes specifically in the ventral region. Furthermore, forced activation of BMP or Wnt signaling represses Shh expression. Our results thus indicate that PcG proteins suppress BMP and Wnt signaling in a region-specific manner and thereby allow proper Shh expression and development of the ventral telencephalon. 1 Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. 2 Laboratory for Developmental Genetics, RIKEN Center for Integrative Medical Sciences (RIKEN-IMS), 1-7-22, Suehiro-cho, Tsurumi-ku, Yokohama 230-0045, Japan. -

Fibroblasts from the Human Skin Dermo-Hypodermal Junction Are
cells Article Fibroblasts from the Human Skin Dermo-Hypodermal Junction are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling Valérie Haydont 1,*, Véronique Neiveyans 1, Philippe Perez 1, Élodie Busson 2, 2 1, 3,4,5,6, , Jean-Jacques Lataillade , Daniel Asselineau y and Nicolas O. Fortunel y * 1 Advanced Research, L’Oréal Research and Innovation, 93600 Aulnay-sous-Bois, France; [email protected] (V.N.); [email protected] (P.P.); [email protected] (D.A.) 2 Department of Medical and Surgical Assistance to the Armed Forces, French Forces Biomedical Research Institute (IRBA), 91223 CEDEX Brétigny sur Orge, France; [email protected] (É.B.); [email protected] (J.-J.L.) 3 Laboratoire de Génomique et Radiobiologie de la Kératinopoïèse, Institut de Biologie François Jacob, CEA/DRF/IRCM, 91000 Evry, France 4 INSERM U967, 92260 Fontenay-aux-Roses, France 5 Université Paris-Diderot, 75013 Paris 7, France 6 Université Paris-Saclay, 78140 Paris 11, France * Correspondence: [email protected] (V.H.); [email protected] (N.O.F.); Tel.: +33-1-48-68-96-00 (V.H.); +33-1-60-87-34-92 or +33-1-60-87-34-98 (N.O.F.) These authors contributed equally to the work. y Received: 15 December 2019; Accepted: 24 January 2020; Published: 5 February 2020 Abstract: Human skin dermis contains fibroblast subpopulations in which characterization is crucial due to their roles in extracellular matrix (ECM) biology. -
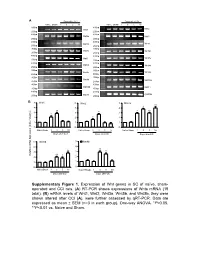
Supplementary Figure 1. Expression of Wnt Genes in SC of Naïve
A Days after CCI Days after CCI Naive Sham 1 3 5 10 Naive Sham 1 3 5 10 500bp 400bp Wnt1 Wnt2 250bp 250bp 400bp 400bp Wnt2b Wnt3 250bp 250bp 400bp 400bp Wnt3a Wnt4 250bp 250bp 300bp 400bp Wnt5a Wnt5b 200bp 250bp 400bp 400bp Wnt6 Wnt7a 250bp 250bp 400bp 400bp Wnt7b Wnt8a 250bp 250bp 400bp 350bp Wnt8b Wnt9a 250bp 200bp 400bp 400bp Wnt9b Wnt10a 250bp 250bp 400bp 400bp Wnt10b Wnt11 250bp 250bp 400bp 400bp Wnt16 GAPDH 250bp 250bp B 5 Wnt1 5 Wnt2 5 Wnt3a ** 4 4 4 * ** ** 3 3 * 3 * * 2 2 * 2 1 1 1 0 0 0 NaïveSham 1 3 5 10 Naïve Sham 1 3 5 10 Naïve Sham 1 3 5 10 Days after CCI Days after CCI Days after CCI 5 Wnt5b 5 Wnt8b 4 4 ** 3 ** 3 ** Relative mRNA Expression (fold of change) ** 2 * 2 1 1 0 0 NaïveSham 1 3 5 10 NaïveSham 1 3 5 10 Days after CCI Days after CCI Supplementary Figure 1 A B Negative Weak Moderate Strong Negative Weak Moderate Strong Large-sized cells 100% 100% 80% 80% 60% 60% 40% 20% 40% 0% 20% Sham CCI-1d CCI-14d Medium-sized cells Wnt3a immunoreactivity cells 0% 100% CGRP(+) IB4(+) 80% Small cells 60% 40% 20% 0% Sham CCI-1d CCI-14d Wnt3a immunoreactivity cells Small cells 100% 80% 60% 40% 20% 0% Sham CCI-1d CCI-14d Fig. S2 4 Fz1 4 Fz3 4 Fz4 4 Fz5 ** 3 3 ** 3 3 2 ** 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1 5 10 NaïveSham 1 5 10 Days after CCI Days after CCI Days after CCI Days after CCI 4 Fz6 4 Fz7 4 Fz8 4 Fz9 (Fold of Change) of (Fold 3 3 3 ** 3 Relative mRNA Expression Expression mRNA Relative ** 2 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1d 5d 10d NaïveSham 1 -

Molecular Discrimination of Cutaneous Squamous Cell Carcinoma from Actinic Keratosis and Normal Skin
Modern Pathology (2011) 24, 963–973 & 2011 USCAP, Inc. All rights reserved 0893-3952/11 $32.00 963 Molecular discrimination of cutaneous squamous cell carcinoma from actinic keratosis and normal skin Seong Hui Ra, Xinmin Li and Scott Binder Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA Actinic keratosis is widely believed to be a neoplastic lesion and a precursor to invasive squamous cell carcinoma. However, there has been some debate as to whether actinic keratosis is in fact actually squamous cell carcinoma and should be treated as such. As the clinical management and prognosis of patients is widely held to be different for each of these lesions, our goal was to identify unique gene signatures using DNA microarrays to discriminate among normal skin, actinic keratosis, and squamous cell carcinoma, and examine the molecular pathways of carcinogenesis involved in the progression from normal skin to squamous cell carcinoma. Formalin-fixed and paraffin-embedded blocks of skin: five normal skins (pooled), six actinic keratoses, and six squamous cell carcinomas were retrieved. The RNA was extracted and amplified. The labeled targets were hybridized to the Affymetrix human U133plus2.0 array and the acquisition and initial quantification of array images were performed using the GCOS (Affymetrix). The subsequent data analyses were performed using DNA-Chip Analyzer and Partek Genomic Suite 6.4. Significant differential gene expression (42 fold change, Po0.05) was seen with 382 differentially expressed genes between squamous cell carcinoma and normal skin, 423 differentially expressed genes between actinic keratosis and normal skin, and 9 differentially expressed genes between actinic keratosis and squamous cell carcinoma.