A Canonical to Non-Canonical Wnt Signalling Switch in Haematopoietic Stem-Cell Ageing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WNT4 and WNT3A Activate Cell Autonomous Wnt Signaling Independent of Secretion
bioRxiv preprint doi: https://doi.org/10.1101/333906; this version posted September 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Running Title: Secretion-independent Wnt signaling Research article WNT4 and WNT3A activate cell autonomous Wnt signaling independent of secretion Deviyani M. Rao1, Rebecca L. Ferguson1, Tomomi M. Yamamoto2, Benjamin G. Bitler2, Matthew J. Sikora1 Affiliation: 1Dept. of Pathology, 2Dept. of Obstetrics and Gynecology, University of Colorado Anschutz Medical Campus Corresponding author: Matthew J. Sikora, PhD.; Mail Stop 8104, Research Complex 1 South, Room 5117, 12801 E. 17th Ave.; Aurora, CO 80045. Tel: (303)724-4301; Fax: (303)724-3712; email: [email protected]. Twitter: @mjsikora Funding This work was supported by R00 CA193734 (MJS) and R00 CA194318 (BGB) from the National Institutes of Health, and by a grant from the Cancer League of Colorado, Inc (MJS). Authors' contributions DMR and MJS conceived of the project and experiments. DMR, RLF, and MJS designed and performed experiments. RLF, DMR, and TMY developed models for the project. DMR, RLF, BGB, and MJS contributed to data analysis and interpretation. DMR wrote the draft manuscript; all authors read and revised the manuscript, and have read and approved of this version of the manuscript. bioRxiv preprint doi: https://doi.org/10.1101/333906; this version posted September 14, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response
International Journal of Molecular Sciences Review Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response Casey D. Stefanski 1,2 and Jenifer R. Prosperi 1,2,3,* 1 Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46617, USA; [email protected] 2 Mike and Josie Harper Cancer Research Institute, South Bend, IN 46617, USA 3 Department of Biochemistry and Molecular Biology, Indiana University School of Medicine-South Bend, South Bend, IN 46617, USA * Correspondence: [email protected]; Tel.: +1-574-631-4002 Received: 30 September 2020; Accepted: 20 October 2020; Published: 22 October 2020 Abstract: Resistance to chemotherapy occurs through mechanisms within the epithelial tumor cells or through interactions with components of the tumor microenvironment (TME). Chemoresistance and the development of recurrent tumors are two of the leading factors of cancer-related deaths. The Adenomatous Polyposis Coli (APC) tumor suppressor is lost in many different cancers, including colorectal, breast, and prostate cancer, and its loss correlates with a decreased overall survival in cancer patients. While APC is commonly known for its role as a negative regulator of the WNT pathway, APC has numerous binding partners and functional roles. Through APC’s interactions with DNA repair proteins, DNA replication proteins, tubulin, and other components, recent evidence has shown that APC regulates the chemotherapy response in cancer cells. In this review article, we provide an overview of some of the cellular processes in which APC participates and how they impact chemoresistance through both epithelial- and TME-derived mechanisms. Keywords: adenomatous polyposis coli; chemoresistance; WNT signaling 1. -

The Sec14-Like Phosphatidylinositol Transfer Proteins Sec14l3/SEC14L2
RESEARCH ARTICLE The Sec14-like phosphatidylinositol transfer proteins Sec14l3/SEC14L2 act as GTPase proteins to mediate Wnt/Ca2+ signaling Bo Gong, Weimin Shen, Wanghua Xiao, Yaping Meng, Anming Meng*, Shunji Jia* State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, China Abstract The non-canonical Wnt/Ca2+ signaling pathway plays important roles in embryonic development, tissue formation and diseases. However, it is unclear how the Wnt ligand-stimulated, G protein-coupled receptor Frizzled activates phospholipases for calcium release. Here, we report that the zebrafish/human phosphatidylinositol transfer protein Sec14l3/SEC14L2 act as GTPase proteins to transduce Wnt signals from Frizzled to phospholipase C (PLC). Depletion of sec14l3 attenuates Wnt/Ca2+ responsive activity and causes convergent and extension (CE) defects in zebrafish embryos. Biochemical analyses in mammalian cells indicate that Sec14l3-GDP forms complex with Frizzled and Dishevelled; Wnt ligand binding of Frizzled induces translocation of Sec14l3 to the plasma membrane; and then Sec14l3-GTP binds to and activates phospholipase Cd4a (Plcd4a); subsequently, Plcd4a initiates phosphatidylinositol-4,5-bisphosphate (PIP2) signaling, ultimately stimulating calcium release. Furthermore, Plcd4a can act as a GTPase-activating protein to accelerate the hydrolysis of Sec14l3-bound GTP to GDP. Our data provide a new insight into GTPase protein-coupled Wnt/Ca2+ signaling transduction. DOI: 10.7554/eLife.26362.001 *For correspondence: mengam@ mail.tsinghua.edu.cn (AM); jiasj@ mail.tsinghua.edu.cn (SJ) Competing interests: The Introduction authors declare that no Wnt ligands, a large family of secreted lipoglycoproteins, control a large number of developmental competing interests exist. -

Activation of Thewnt–Яcatenin Pathway in a Cell Population on The
The Journal of Neuroscience, September 5, 2007 • 27(36):9757–9768 • 9757 Development/Plasticity/Repair Activation of the Wnt–Catenin Pathway in a Cell Population on the Surface of the Forebrain Is Essential for the Establishment of Olfactory Axon Connections Ambra A. Zaghetto,1 Sara Paina,1 Stefano Mantero,1 Natalia Platonova,1 Paolo Peretto,2 Serena Bovetti,2,3 Adam Puche,3 Stefano Piccolo,4 and Giorgio R. Merlo1 1Dulbecco Telethon Institute-Consiglio Nazionale delle Ricerche Institute for Biomedical Technologies Milano, 20090 Segrate, Italy, 2Department of Animal and Human Biology, University of Torino, 10123 Torino, Italy, 3Department of Anatomy and Neurobiology, School of Medicine, University of Maryland, Baltimore, Maryland 21201, and 4Department of Histology, Microbiology, and Medical Biotechnologies, School of Medicine, University of Padova, 35122 Padova, Italy A variety of signals governing early extension, guidance, and connectivity of olfactory receptor neuron (ORN) axons has been identified; however, little is known about axon–mesoderm and forebrain (FB)–mesoderm signals. Using Wnt–catenin reporter mice, we identify a novel Wnt-responsive resident cell population, located in a Frizzled7 expression domain at the surface of the embryonic FB, along the trajectory of incoming ORN axons. Organotypic slice cultures that recapitulate olfactory-associated Wnt–catenin activation show that the catenin response depends on a placode-derived signal(s). Likewise, in Dlx5Ϫ/Ϫ embryos, in which the primary connections fail to form, Wnt–catenin response on the surface of the FB is strongly reduced. The olfactory placode expresses a number of catenin- activating Wnt genes, and the Frizzled7 receptor transduces the “canonical” Wnt signal; using Wnt expression plasmids we show that Wnt5a and Wnt7b are sufficient to rescue catenin activation in the absence of incoming axons. -

Tsukushi Functions As a Wnt Signaling Inhibitor by Competing with Wnt2b for Binding to Transmembrane Protein Frizzled4
Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4 Kunimasa Ohtaa,b,1,2, Ayako Itoa,c,1, Sei Kuriyamaa,d,3, Giuseppe Lupoe,f, Mitsuko Kosakag,4, Shin-ichi Ohnumah, Shinichi Nakagawai, and Hideaki Tanakaa,c,d aDepartment of Developmental Neurobiology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto 860-8556, Japan; bPrecursory Research for Embryonic Science and Technology, Japan Science and Technology Agency, Saitama 332-0012, Japan; cGlobal Center of Excellence, Kumamoto University, Kumamoto 860-8556, Japan; d21st Century Center of Excellence, Kumamoto University, Kumamoto 860-8556, Japan; eDepartment of Biology and Biotechnology “C. Darwin,” University of Rome “La Sapienza,” 00185 Rome, Italy; fIstituto Pasteur–Fondazione Cenci Bolognetti, 00185, Rome, Italy; gRIKEN Center for Developmental Biology, Kobe 650-0047, Japan; hInstitute of Ophthalmology, University College London, London EC1V 9EL, United Kingdom; and IRIKEN Advanced Science Institute, Nakagawa RNA Biology Laboratory, Saitama 351-0198, Japan Edited* by Lynn T. Landmesser, Case Western Reserve University, Cleveland, OH, and approved July 8, 2011 (received for review January 11, 2011) The Wnt signaling pathway is essential for the development of We previously described the isolation of Tsukushi (TSK) protein diverse tissues during embryogenesis. Signal transduction is acti- isoforms (13), soluble molecules belonging to the small leucine- vated by the binding of Wnt proteins to the type I receptor low- rich proteoglycan (SLRP) family (14), and showed that they work density lipoprotein receptor–related protein 5/6 and the seven-pass as extracellular modulators of pivotal signaling cascades during transmembrane protein Frizzled (Fzd), which contains a Wnt- early embryonic development in chicks and frogs (13, 15–17). -

Hippocampus Formation: an Intriguing Collaboration Henk Roelink
bb10g06.qxd 04/03/2000 01:10 Page R279 Dispatch R279 Hippocampus formation: An intriguing collaboration Henk Roelink Recent genetic studies have shown that the signalling temporal lobes are formed. In an adult rodent, the hip- factor Wnt3a is required for formation of the pocampus is consequently still found close to the dorsal hippocampus; the developmental consequences of Wnt midline where it is initially formed (Figure 1). signalling in the hippocampus are mediated by multiple HMG-box transcription factors, with LEF-1 being It is clear that Wnt family members are required for the required just for formation of the dentate gyrus. development of many embryonic structures, functions mediated by their effects on fundamental processes such Address: Department of Biological Structure and Center for Developmental Biology, University of Washington, Box 357420, as cell proliferation, differentiation, survival or mainte- Seattle, Washington 98117-7420, USA. nance. Obtaining a clear picture of how each Wnt acts is E-mail: [email protected] complicated by the relatively large size of the family, which has at least 18 members in amniotes [3]. Analyses of Current Biology 2000, 10:R279–R281 mice lacking one or several Wnt genes have revealed some 0960-9822/00/$ – see front matter remarkable phenotypes, often characterized by failure of © 2000 Elsevier Science Ltd. All rights reserved. induction of very specific anatomical structures. Wnt3A is no exception, and although several papers have been pub- Inductive interactions are fundamental to the formation of lished already on the more obvious defects of Wnt3a all brain structures. and signalling by molecules of the knockout mice, a recent paper from McMahon and col- Wingless/Wnt family is known to play a role in many of leagues [1] has reported a very interesting defect in the them. -

A Membrane-Associated Β-Catenin/Oct4 Complex Correlates
DEVELOPMENT AND STEM CELLS RESEARCH ARTICLE 1171 Development 140, 1171-1183 (2013) doi:10.1242/dev.085654 © 2013. Published by The Company of Biologists Ltd A membrane-associated β-catenin/Oct4 complex correlates with ground-state pluripotency in mouse embryonic stem cells Fernando Faunes1,*, Penelope Hayward1,*, Silvia Muñoz Descalzo1, Sujash S. Chatterjee2, Tina Balayo1, Jamie Trott1, Andrew Christoforou1,3, Anna Ferrer-Vaquer4, Anna-Katerina Hadjantonakis4, Ramanuj Dasgupta2 and Alfonso Martinez Arias1,‡ SUMMARY The maintenance of pluripotency in mouse embryonic stem cells (mESCs) relies on the activity of a transcriptional network that is fuelled by the activity of three transcription factors (Nanog, Oct4 and Sox2) and balanced by the repressive activity of Tcf3. Extracellular signals modulate the activity of the network and regulate the differentiation capacity of the cells. Wnt/β-catenin signaling has emerged as a significant potentiator of pluripotency: increases in the levels of β-catenin regulate the activity of Oct4 and Nanog, and enhance pluripotency. A recent report shows that β-catenin achieves some of these effects by modulating the activity of Tcf3, and that this effect does not require its transcriptional activation domain. Here, we show that during self-renewal there is negligible transcriptional activity of β-catenin and that this is due to its tight association with membranes, where we find it in a complex with Oct4 and E-cadherin. Differentiation triggers a burst of Wnt/β-catenin transcriptional activity that coincides with the disassembly of the complex. Our results establish that β-catenin, but not its transcriptional activity, is central to pluripotency acting through a β- catenin/Oct4 complex. -

Beta;-Catenin Signaling in Rhabdomyosarcoma
Laboratory Investigation (2013) 93, 1090–1099 & 2013 USCAP, Inc All rights reserved 0023-6837/13 Characterization of Wnt/b-catenin signaling in rhabdomyosarcoma Srinivas R Annavarapu1,8,9, Samantha Cialfi2,9, Carlo Dominici2,3, George K Kokai4, Stefania Uccini5, Simona Ceccarelli6, Heather P McDowell2,3,7 and Timothy R Helliwell1 Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and accounts for about 5% of all malignant paediatric tumours. b-Catenin, a multifunctional nuclear transcription factor in the canonical Wnt signaling pathway, is active in myogenesis and embryonal somite patterning. Dysregulation of Wnt signaling facilitates tumour invasion and metastasis. This study characterizes Wnt/b-catenin signaling and functional activity in paediatric embryonal and alveolar RMS. Immunohistochemical assessment of paraffin-embedded tissues from 44 RMS showed b-catenin expression in 26 cases with cytoplasmic/membranous expression in 9/14 cases of alveolar RMS, and 15/30 cases of embryonal RMS, whereas nuclear expression was only seen in 2 cases of embryonal RMS. The potential functional significance of b-catenin expression was tested in four RMS cell lines, two derived from embryonal (RD and RD18) RMS and two from alveolar (Rh4 and Rh30) RMS. Western blot analysis demonstrated the expression of Wnt-associated proteins including b-catenin, glycogen synthase kinase-3b, disheveled, axin-1, naked, LRP-6 and cadherins in all cell lines. Cell fractionation and immunofluorescence studies of the cell lines (after stimulation by human recombinant Wnt3a) showed reduced phosphorylation of b-catenin, stabilization of the active cytosolic form and nuclear translocation of b-catenin. Reporter gene assay demonstrated a T-cell factor/lymphoid-enhancing factor-mediated transactivation in these cells. -

Control of Wnt5b Secretion by Wntless Modulates Chondrogenic Cell Proliferation Through Fine-Tuning Fgf3 Expression Bo-Tsung Wu1,2, Shih-Hsien Wen1,2, Sheng-Ping L
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 2328-2339 doi:10.1242/jcs.167403 RESEARCH ARTICLE Control of Wnt5b secretion by Wntless modulates chondrogenic cell proliferation through fine-tuning fgf3 expression Bo-Tsung Wu1,2, Shih-Hsien Wen1,2, Sheng-Ping L. Hwang3, Chang-Jen Huang1,2 and Yung-Shu Kuan1,2,4,* ABSTRACT activities to achieve the proper proliferation, differentiation or Wnts and Fgfs regulate various tissues development in migration responses is still relatively limited. β vertebrates. However, how regional Wnt or Fgf activities are In vertebrates, the -catenin-mediated canonical and the non- established and how they interact in any given developmental canonical Wnt signaling pathways have both been shown to be event is elusive. Here, we investigated the Wnt-mediated involved in the processes of craniofacial skeleton formation. In craniofacial cartilage development in zebrafish and found that mice, previous observations have indicated that Wnts can either fgf3 expression in the pharyngeal pouches is differentially reduced stimulate chondrogenesis by promoting survival and differentiation along the anteroposterior axis in wnt5b mutants and wntless (wls) of migrating neural crest cells (NCCs), or inhibit chondrogenesis by morphants, but its expression is normal in wnt9a and wnt11 repressing BMP2-induced chondrocyte gene expression, depending morphants. Introducing fgf3 mRNAs rescued the cartilage defects on the developmental stage and the local tissue context (Brault et al., in Wnt5b- and Wls-deficient larvae. In wls morphants, endogenous 2001; Liu et al., 2008; Reinhold et al., 2006; Yang et al., 2003). In Wls expression is not detectable but maternally deposited Wls is zebrafish, wnt4a and wnt11r have been shown to regulate the present in eggs, which might account for the lack of axis defects in formation of pharyngeal pouches, whereas wnt5b, wnt9a and wnt11 wls morphants. -

Wnt3a: Functions and Implications in Cancer Sha He1,2, Yi Lu1,2, Xia Liu1,2, Xin Huang1,2, Evan T
He et al. Chin J Cancer (2015) 34:50 DOI 10.1186/s40880-015-0052-4 REVIEW Open Access Wnt3a: functions and implications in cancer Sha He1,2, Yi Lu1,2, Xia Liu1,2, Xin Huang1,2, Evan T. Keller3, Chao‑Nan Qian4* and Jian Zhang1,2,3* Abstract Wnt3a, one of Wnt family members, plays key roles in regulating pleiotropic cellular functions, including self-renewal, proliferation, differentiation, and motility. Accumulating evidence has suggested that Wnt3a promotes or suppresses tumor progression via the canonical Wnt signaling pathway depending on cancer type. In addition, the roles of Wnt3a signaling can be inhibited by multiple proteins or chemicals. Herein, we summarize the latest findings on Wnt3a as an important therapeutic target in cancer. Keywords: Wnt3a, The Wnt signaling pathway, Cancer Background: the Wnt signaling pathway cell polarity pathway (Wnt-PCP pathway) and the Wnt- The Wnt gene was first identified in 1982 and is named calcium pathway (Wnt-Ca2+ pathway) [5]. According to the Int gene in mice [1]. A subsequent study reported the characteristics of its functions, the Wnt family can that the Int gene and wingless gene in Drosophila were also be divided into two categories: Wnt1 and Wnt5a. homologous genes [2]. Therefore, both genes were rec- The Wnt1 category includes Wnt1, Wnt2, Wnt2b, Wnt3, ognized as the Wnt gene [3]. The Wnt family comprises Wnt3a, Wnt7a, Wnt8, Wnt8b, and Wnt10a, which are 19 human proteins, including Wnt1, Wnt2, Wnt2b involved in the canonical signaling pathway, whereas (Wnt13), Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt4, Wnt5a, and Wnt11 belong to the Wnt5a category Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt9a (Wnt14), Wnt9b and activate the noncanonical signaling pathway. -

Induction of Apoptosis Increases Expression of Non-Canonical WNT Genes in Myeloid Leukemia Cell Lines
1563-1569 7/11/07 19:04 Page 1563 ONCOLOGY REPORTS 18: 1563-1569, 2007 Induction of apoptosis increases expression of non-canonical WNT genes in myeloid leukemia cell lines HAKKI OGUN SERCAN1, MELEK PEHLIVAN1, OZLENEN SIMSEK1, HALIL ATES2 and ZEYNEP SERCAN1 Depatments of 1Medical Biology and Genetics, 2Hematology/Oncology, Dokuz Eylul University Faculty of Medicine, Izmir, Turkey Received May 3, 2007; Accepted July 20, 2007 Abstract. With the aim of determining the differential and cyclin D. The presence or exclusion of co-receptors (low expression of WNT and FZD genes, before and after density lipoprotein receptor-related protein 5 and 6-LRP5/6) induction of apoptosis in BCR-ABL positive cells, we treated or secreted Wnt antagonists (soluble frizzled related proteins the myeloid cell line K562 and control cell line HL60 with - sFRPs- and Dickkopf proteins) increases the complexity of imatinib mesylate and etoposide, and analyzed relative the molecular mechanisms underlying cellular outcomes. mRNA expression levels of WNT, FZD and sFRP genes Non-canonical pathway activation is independent of ß- under normal and apoptotic conditions by real-time RT-PCR. catenin. Two non-canonical pathways have also been defined: We observed marked increase in mRNA levels of FZD4, the planar cell polarity (PCP) and the Wnt/Ca2+ pathways (3-6). FZD5, FZD7 and WNT5b, correlating with apoptotic activity There is an overlap of proposed Wnt and Fzd proteins and and independent of the agent or cell line used. Our results secondary messengers participating in these non-canonical suggest the involvement of non-canonical Wnt signaling in pathways, complicating further the interpretation of experi- executing programmed cell death in myeloid cell lines. -
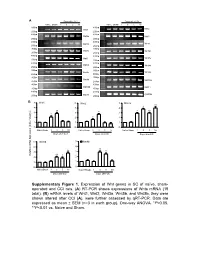
Supplementary Figure 1. Expression of Wnt Genes in SC of Naïve
A Days after CCI Days after CCI Naive Sham 1 3 5 10 Naive Sham 1 3 5 10 500bp 400bp Wnt1 Wnt2 250bp 250bp 400bp 400bp Wnt2b Wnt3 250bp 250bp 400bp 400bp Wnt3a Wnt4 250bp 250bp 300bp 400bp Wnt5a Wnt5b 200bp 250bp 400bp 400bp Wnt6 Wnt7a 250bp 250bp 400bp 400bp Wnt7b Wnt8a 250bp 250bp 400bp 350bp Wnt8b Wnt9a 250bp 200bp 400bp 400bp Wnt9b Wnt10a 250bp 250bp 400bp 400bp Wnt10b Wnt11 250bp 250bp 400bp 400bp Wnt16 GAPDH 250bp 250bp B 5 Wnt1 5 Wnt2 5 Wnt3a ** 4 4 4 * ** ** 3 3 * 3 * * 2 2 * 2 1 1 1 0 0 0 NaïveSham 1 3 5 10 Naïve Sham 1 3 5 10 Naïve Sham 1 3 5 10 Days after CCI Days after CCI Days after CCI 5 Wnt5b 5 Wnt8b 4 4 ** 3 ** 3 ** Relative mRNA Expression (fold of change) ** 2 * 2 1 1 0 0 NaïveSham 1 3 5 10 NaïveSham 1 3 5 10 Days after CCI Days after CCI Supplementary Figure 1 A B Negative Weak Moderate Strong Negative Weak Moderate Strong Large-sized cells 100% 100% 80% 80% 60% 60% 40% 20% 40% 0% 20% Sham CCI-1d CCI-14d Medium-sized cells Wnt3a immunoreactivity cells 0% 100% CGRP(+) IB4(+) 80% Small cells 60% 40% 20% 0% Sham CCI-1d CCI-14d Wnt3a immunoreactivity cells Small cells 100% 80% 60% 40% 20% 0% Sham CCI-1d CCI-14d Fig. S2 4 Fz1 4 Fz3 4 Fz4 4 Fz5 ** 3 3 ** 3 3 2 ** 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1 5 10 NaïveSham 1 5 10 Days after CCI Days after CCI Days after CCI Days after CCI 4 Fz6 4 Fz7 4 Fz8 4 Fz9 (Fold of Change) of (Fold 3 3 3 ** 3 Relative mRNA Expression Expression mRNA Relative ** 2 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1d 5d 10d NaïveSham 1