The Sec14-Like Phosphatidylinositol Transfer Proteins Sec14l3/SEC14L2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activation of Thewnt–Яcatenin Pathway in a Cell Population on The
The Journal of Neuroscience, September 5, 2007 • 27(36):9757–9768 • 9757 Development/Plasticity/Repair Activation of the Wnt–Catenin Pathway in a Cell Population on the Surface of the Forebrain Is Essential for the Establishment of Olfactory Axon Connections Ambra A. Zaghetto,1 Sara Paina,1 Stefano Mantero,1 Natalia Platonova,1 Paolo Peretto,2 Serena Bovetti,2,3 Adam Puche,3 Stefano Piccolo,4 and Giorgio R. Merlo1 1Dulbecco Telethon Institute-Consiglio Nazionale delle Ricerche Institute for Biomedical Technologies Milano, 20090 Segrate, Italy, 2Department of Animal and Human Biology, University of Torino, 10123 Torino, Italy, 3Department of Anatomy and Neurobiology, School of Medicine, University of Maryland, Baltimore, Maryland 21201, and 4Department of Histology, Microbiology, and Medical Biotechnologies, School of Medicine, University of Padova, 35122 Padova, Italy A variety of signals governing early extension, guidance, and connectivity of olfactory receptor neuron (ORN) axons has been identified; however, little is known about axon–mesoderm and forebrain (FB)–mesoderm signals. Using Wnt–catenin reporter mice, we identify a novel Wnt-responsive resident cell population, located in a Frizzled7 expression domain at the surface of the embryonic FB, along the trajectory of incoming ORN axons. Organotypic slice cultures that recapitulate olfactory-associated Wnt–catenin activation show that the catenin response depends on a placode-derived signal(s). Likewise, in Dlx5Ϫ/Ϫ embryos, in which the primary connections fail to form, Wnt–catenin response on the surface of the FB is strongly reduced. The olfactory placode expresses a number of catenin- activating Wnt genes, and the Frizzled7 receptor transduces the “canonical” Wnt signal; using Wnt expression plasmids we show that Wnt5a and Wnt7b are sufficient to rescue catenin activation in the absence of incoming axons. -

Control of Wnt5b Secretion by Wntless Modulates Chondrogenic Cell Proliferation Through Fine-Tuning Fgf3 Expression Bo-Tsung Wu1,2, Shih-Hsien Wen1,2, Sheng-Ping L
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 2328-2339 doi:10.1242/jcs.167403 RESEARCH ARTICLE Control of Wnt5b secretion by Wntless modulates chondrogenic cell proliferation through fine-tuning fgf3 expression Bo-Tsung Wu1,2, Shih-Hsien Wen1,2, Sheng-Ping L. Hwang3, Chang-Jen Huang1,2 and Yung-Shu Kuan1,2,4,* ABSTRACT activities to achieve the proper proliferation, differentiation or Wnts and Fgfs regulate various tissues development in migration responses is still relatively limited. β vertebrates. However, how regional Wnt or Fgf activities are In vertebrates, the -catenin-mediated canonical and the non- established and how they interact in any given developmental canonical Wnt signaling pathways have both been shown to be event is elusive. Here, we investigated the Wnt-mediated involved in the processes of craniofacial skeleton formation. In craniofacial cartilage development in zebrafish and found that mice, previous observations have indicated that Wnts can either fgf3 expression in the pharyngeal pouches is differentially reduced stimulate chondrogenesis by promoting survival and differentiation along the anteroposterior axis in wnt5b mutants and wntless (wls) of migrating neural crest cells (NCCs), or inhibit chondrogenesis by morphants, but its expression is normal in wnt9a and wnt11 repressing BMP2-induced chondrocyte gene expression, depending morphants. Introducing fgf3 mRNAs rescued the cartilage defects on the developmental stage and the local tissue context (Brault et al., in Wnt5b- and Wls-deficient larvae. In wls morphants, endogenous 2001; Liu et al., 2008; Reinhold et al., 2006; Yang et al., 2003). In Wls expression is not detectable but maternally deposited Wls is zebrafish, wnt4a and wnt11r have been shown to regulate the present in eggs, which might account for the lack of axis defects in formation of pharyngeal pouches, whereas wnt5b, wnt9a and wnt11 wls morphants. -

A Canonical to Non-Canonical Wnt Signalling Switch in Haematopoietic Stem-Cell Ageing
LETTER doi:10.1038/nature12631 A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing Maria Carolina Florian1, Kalpana J. Nattamai2, Karin Do¨rr1, Gina Marka1, Bettina U¨berle1, Virag Vas1, Christina Eckl3, Immanuel Andra¨4, Matthias Schiemann4, Robert A. J. Oostendorp3, Karin Scharffetter-Kochanek1, Hans Armin Kestler5, Yi Zheng2 & Hartmut Geiger1,2 Many organs with a high cell turnover (for example, skin, intestine LT-HSCs presented with a reduced level and primarily cytoplasmic loca- and blood) are composed of short-lived cells that require continuous lization of b-catenin (Fig. 1d, e and Supplementary Video 3). Reduced replenishment by somatic stem cells1,2. Ageing results in the inability levels of b-catenin upon ageing were specific to the LT-HSC compart- of these tissues to maintain homeostasis and it is believed that somatic ment, as more differentiated LKs (Lin2c-Kit1Sca-12 cells), LSKs stem-cell ageing is one underlying cause of tissue attrition with age (Lin2Sca-11c-Kit1 cells), lymphoid-primed multipotent progenitors or age-related diseases. Ageing of haematopoietic stem cells (HSCs) (LMPPs; Lin2c-Kit1Sca-12CD341Flk21 cells) and short-term (ST)- is associated with impaired haematopoiesis in the elderly3–6. Despite HSCs (Lin2c-Kit1Sca-12CD341Flk22 cells) (Extended Data Fig. 1h) a large amount of data describing the decline of HSC function on showed similar levels of b-catenin upon ageing (Fig. 1g and Extended ageing, the molecular mechanisms of this process remain largely Data Fig. 1i). Axin2 (an established direct downstream target of cano- unknown, which precludes rational approaches to attenuate stem- nical Wnt signalling9,15) transcript levels in aged LT-HSCs were mark- cell ageing. -

Induction of Apoptosis Increases Expression of Non-Canonical WNT Genes in Myeloid Leukemia Cell Lines
1563-1569 7/11/07 19:04 Page 1563 ONCOLOGY REPORTS 18: 1563-1569, 2007 Induction of apoptosis increases expression of non-canonical WNT genes in myeloid leukemia cell lines HAKKI OGUN SERCAN1, MELEK PEHLIVAN1, OZLENEN SIMSEK1, HALIL ATES2 and ZEYNEP SERCAN1 Depatments of 1Medical Biology and Genetics, 2Hematology/Oncology, Dokuz Eylul University Faculty of Medicine, Izmir, Turkey Received May 3, 2007; Accepted July 20, 2007 Abstract. With the aim of determining the differential and cyclin D. The presence or exclusion of co-receptors (low expression of WNT and FZD genes, before and after density lipoprotein receptor-related protein 5 and 6-LRP5/6) induction of apoptosis in BCR-ABL positive cells, we treated or secreted Wnt antagonists (soluble frizzled related proteins the myeloid cell line K562 and control cell line HL60 with - sFRPs- and Dickkopf proteins) increases the complexity of imatinib mesylate and etoposide, and analyzed relative the molecular mechanisms underlying cellular outcomes. mRNA expression levels of WNT, FZD and sFRP genes Non-canonical pathway activation is independent of ß- under normal and apoptotic conditions by real-time RT-PCR. catenin. Two non-canonical pathways have also been defined: We observed marked increase in mRNA levels of FZD4, the planar cell polarity (PCP) and the Wnt/Ca2+ pathways (3-6). FZD5, FZD7 and WNT5b, correlating with apoptotic activity There is an overlap of proposed Wnt and Fzd proteins and and independent of the agent or cell line used. Our results secondary messengers participating in these non-canonical suggest the involvement of non-canonical Wnt signaling in pathways, complicating further the interpretation of experi- executing programmed cell death in myeloid cell lines. -
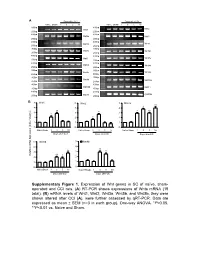
Supplementary Figure 1. Expression of Wnt Genes in SC of Naïve
A Days after CCI Days after CCI Naive Sham 1 3 5 10 Naive Sham 1 3 5 10 500bp 400bp Wnt1 Wnt2 250bp 250bp 400bp 400bp Wnt2b Wnt3 250bp 250bp 400bp 400bp Wnt3a Wnt4 250bp 250bp 300bp 400bp Wnt5a Wnt5b 200bp 250bp 400bp 400bp Wnt6 Wnt7a 250bp 250bp 400bp 400bp Wnt7b Wnt8a 250bp 250bp 400bp 350bp Wnt8b Wnt9a 250bp 200bp 400bp 400bp Wnt9b Wnt10a 250bp 250bp 400bp 400bp Wnt10b Wnt11 250bp 250bp 400bp 400bp Wnt16 GAPDH 250bp 250bp B 5 Wnt1 5 Wnt2 5 Wnt3a ** 4 4 4 * ** ** 3 3 * 3 * * 2 2 * 2 1 1 1 0 0 0 NaïveSham 1 3 5 10 Naïve Sham 1 3 5 10 Naïve Sham 1 3 5 10 Days after CCI Days after CCI Days after CCI 5 Wnt5b 5 Wnt8b 4 4 ** 3 ** 3 ** Relative mRNA Expression (fold of change) ** 2 * 2 1 1 0 0 NaïveSham 1 3 5 10 NaïveSham 1 3 5 10 Days after CCI Days after CCI Supplementary Figure 1 A B Negative Weak Moderate Strong Negative Weak Moderate Strong Large-sized cells 100% 100% 80% 80% 60% 60% 40% 20% 40% 0% 20% Sham CCI-1d CCI-14d Medium-sized cells Wnt3a immunoreactivity cells 0% 100% CGRP(+) IB4(+) 80% Small cells 60% 40% 20% 0% Sham CCI-1d CCI-14d Wnt3a immunoreactivity cells Small cells 100% 80% 60% 40% 20% 0% Sham CCI-1d CCI-14d Fig. S2 4 Fz1 4 Fz3 4 Fz4 4 Fz5 ** 3 3 ** 3 3 2 ** 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1 5 10 NaïveSham 1 5 10 Days after CCI Days after CCI Days after CCI Days after CCI 4 Fz6 4 Fz7 4 Fz8 4 Fz9 (Fold of Change) of (Fold 3 3 3 ** 3 Relative mRNA Expression Expression mRNA Relative ** 2 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1d 5d 10d NaïveSham 1 -

Molecular Discrimination of Cutaneous Squamous Cell Carcinoma from Actinic Keratosis and Normal Skin
Modern Pathology (2011) 24, 963–973 & 2011 USCAP, Inc. All rights reserved 0893-3952/11 $32.00 963 Molecular discrimination of cutaneous squamous cell carcinoma from actinic keratosis and normal skin Seong Hui Ra, Xinmin Li and Scott Binder Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA Actinic keratosis is widely believed to be a neoplastic lesion and a precursor to invasive squamous cell carcinoma. However, there has been some debate as to whether actinic keratosis is in fact actually squamous cell carcinoma and should be treated as such. As the clinical management and prognosis of patients is widely held to be different for each of these lesions, our goal was to identify unique gene signatures using DNA microarrays to discriminate among normal skin, actinic keratosis, and squamous cell carcinoma, and examine the molecular pathways of carcinogenesis involved in the progression from normal skin to squamous cell carcinoma. Formalin-fixed and paraffin-embedded blocks of skin: five normal skins (pooled), six actinic keratoses, and six squamous cell carcinomas were retrieved. The RNA was extracted and amplified. The labeled targets were hybridized to the Affymetrix human U133plus2.0 array and the acquisition and initial quantification of array images were performed using the GCOS (Affymetrix). The subsequent data analyses were performed using DNA-Chip Analyzer and Partek Genomic Suite 6.4. Significant differential gene expression (42 fold change, Po0.05) was seen with 382 differentially expressed genes between squamous cell carcinoma and normal skin, 423 differentially expressed genes between actinic keratosis and normal skin, and 9 differentially expressed genes between actinic keratosis and squamous cell carcinoma. -

Expression Profiles and Prognostic Significance of WNT Family
Bioscience Reports (2020) 40 BSR20194255 https://doi.org/10.1042/BSR20194255 Research Article Expression profiles and prognostic significance of WNT family members in glioma via bioinformatic analysis Anqi Xu1, Huiping Yang2, Kunjie Gao2, Zhengming Zhan1, Zibin Song2, Tengyue Huang3 and Ye Song1 1Department of Neurosurgery, Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong, P.R. China; 2The First Clinical Medical Institute of Southern Medical University, Guangzhou 510515, P.R. China; 3Gannan Medical University, Ganzhou 341000, Jiang xi, P.R. China Correspondence: Ye Song ([email protected]) Aims: The dysregulation and essential role of WNTs in glioma have been widely implicated. However, there is a paucity of literature on the expression status of all the 19 WNTs in glioma. Our study was aimed to evaluate the expression and prognostic values of the 19 WNTs in glioma. Methods: mRNA expression and clinical data were retrieved from the Cancer Genome Atlas (TCGA) database, Chinese Glioma Genome Atlas (CGGA), GTEx and ON- COMINE databases. The 50 frequent neighbor genes of WNT5A and WNT10B were shown with PPI network, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. Results: We found that the mRNA expression of WNT5A was significantly higher in glioma; however, the WNT10B expression was significantly lower in glioma. Fur- thermore, the expression of WNT5A and WNT10B was associated with the clinicopathology of glioma. The survival analysis revealed that the higher expressions of WNT5A and WNT16 were associated poor overall survival (OS) in patients with glioma. Conversely, overexpres- sion of WNT3, WNT5B, and WNT10B was associated with better OS. -

Mycobacterium Tuberculosis of Wnt6 Is Expresse
Wnt6 Is Expressed in Granulomatous Lesions of Mycobacterium tuberculosis−Infected Mice and Is Involved in Macrophage Differentiation and Proliferation This information is current as of September 27, 2021. Kolja Schaale, Julius Brandenburg, Andreas Kispert, Michael Leitges, Stefan Ehlers and Norbert Reiling J Immunol 2013; 191:5182-5195; Prepublished online 11 October 2013; doi: 10.4049/jimmunol.1201819 Downloaded from http://www.jimmunol.org/content/191/10/5182 Supplementary http://www.jimmunol.org/content/suppl/2013/10/11/jimmunol.120181 http://www.jimmunol.org/ Material 9.DC1 References This article cites 60 articles, 23 of which you can access for free at: http://www.jimmunol.org/content/191/10/5182.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 27, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights -

Roles of Wnt Pathway Genes Wls, Wnt9a, Wnt5b, Frzb and Gpc4 in Regulating Convergent-Extension During Zebrafish Palate Morphogenesis Lucie Rochard1, Stefanie D
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 2541-2547 doi:10.1242/dev.137000 RESEARCH REPORT Roles of Wnt pathway genes wls, wnt9a, wnt5b, frzb and gpc4 in regulating convergent-extension during zebrafish palate morphogenesis Lucie Rochard1, Stefanie D. Monica2, Irving T. C. Ling1, Yawei Kong1, Sara Roberson3, Richard Harland2, Marnie Halpern3 and Eric C. Liao1,* ABSTRACT Wnts are highly conserved secreted proteins that share a The Wnt signaling pathway is crucial for tissue morphogenesis, common secretion factor, Wntless (Wls), which is a multi-pass participating in cellular behavior changes, notably during the process transmembrane protein that transports Wnt from the Golgi apparatus of convergent-extension. Interactions between Wnt-secreting and to the cell membrane (Bartscherer and Boutros, 2008; Brugmann receiving cells during convergent-extension remain elusive. We et al., 2007; Franch-Marro et al., 2008; Garcia-Castro et al., 2002; investigated the role and genetic interactions of Wnt ligands and Gleason et al., 2006; Harterink and Korswagen, 2012; Lee et al., their trafficking factors Wls, Gpc4 and Frzb in the context of palate 2008; Najdi et al., 2012; Wodarz and Nusse, 1998). Wnts show high morphogenesis in zebrafish. We describe that the chaperon Wls and hydrophobicity, which limits their free diffusion in the extracellular its ligands Wnt9a and Wnt5b are expressed in the ectoderm, whereas space. Extracellular matrix components, such as Glypican (Gpc4), juxtaposed chondrocytes express Frzb and Gpc4. Using wls, gpc4, ensure their diffusion to neighboring cells, while sFRP (Frzb) frzb, wnt9a and wnt5b mutants, we genetically dissected the Wnt enhances diffusion of Wnt by blocking short-range effects and signals operating between secreting ectoderm and receiving increasing long-range gene responses (Cadigan and Peifer, 2009; chondrocytes. -

A Novel Human Wnt Gene, WNT10B, Maps to 12Q13 and Is Expressed in Human Breast Carcinomas
Oncogene (1997) 14, 1249 ± 1253 1997 Stockton Press All rights reserved 0950 ± 9232/97 $12.00 SHORT REPORT A novel human Wnt gene, WNT10B, maps to 12q13 and is expressed in human breast carcinomas Thuan D Bui1, Julia Rankin2, Kenneth Smith1, Emmanuel L Huguet1, Steve Ruben3, Tom Strachan2, Adrian L Harris1 and Susan Lindsay2 1Growth Factors Group, Imperial Cancer Research Fund, University of Oxford, Institute of Molecular Medicine, John Radclie Hospital, Headington, Oxford OX3 9DU; 2University of Newcastle upon Tyne, Department of Human Genetics, Ridley Building, Newcastle upon Tyne NE1 7RU, UK; 3Human Genome Sciences, Inc., 9620 Medical Center Dr, Suite 300, Rockville, MD 20850 3338, USA Several members of the Wnt gene family have been silent or expressed at low levels in this tissue causes shown to cause mammary tumors in mouse. Using mammary carcinomas. Thus mouse Wnt1, Wnt3, and degenerate primer polymerase chain reaction (PCR) on recently Wnt10b, has been shown to be some of the human genomic DNA, and speci®c PCR of cDNA oncogenes insertionally activated in the process of libraries, we have isolated a WNT gene which has not MMTV induced carcinogenesis (Nusse and Varmus, previously been described in human. The gene is the 1982; Roelink et al., 1990; Lee et al., 1995). human homologue of mouse Wnt10b, recently shown to Furthermore, murine Wnt1, Wnt2, Wnt3a, Wnt5b, be one of the oncogenes cooperating with FGF3 in the Wnt7a and Wnt7b but not Wnt4, Wnt5a and Wnt6 development of mouse mammary tumour virus (MMTV) have been shown to transform mouse epithelial cells in induced mouse mammary carcinomas. -

Wnt8b Regulates Myo Broblast Differentiation of Lung-Resident Mesenchymal Stem Cells Via the Activation of Wnt/Β-Catenin Signal
Wnt8b Regulates Myobroblast Differentiation of Lung-Resident Mesenchymal Stem Cells via the Activation of Wnt/β-catenin Signaling in Pulmonary Fibrogenesis Chaowen Shi Nanjing University Medical School Xiang Chen Nanjing University Medical School Wenna Yin Nanjing University Medical School Jiwei Hou Nanjing University Medical School Zhaorui Sun Nanjing University Medical School Clinical College: East Region Military Command General Hospital Xiaodong Han ( [email protected] ) Nanjing University https://orcid.org/0000-0003-4512-3699 Research Keywords: Lung resident mesenchymal stem cell, Wnt8b, Wnt/β-catenin signaling, Idiopathic pulmonary brosis Posted Date: May 6th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-466908/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/19 Abstract Background Idiopathic pulmonary brosis (IPF) is a chronic, progressive, and fatal lung disease that is characterized by enhanced changes in stem cell differentiation and broblast proliferation. Lung resident mesenchymal stem cells (LR-MSCs) are important regulators of pathophysiological processes including tissue repair and inammation, and evidence suggests that this cell population also plays an essential role in brosis. Our previous study demonstrated that Wnt/β-catenin signaling is aberrantly activated in the lungs of bleomycin-treated mice and induces myobroblast differentiation of LR-MSCs. However, the underlying correlation between LR-MSCs and the Wnt/β-catenin signaling remains poorly understood. Methods We used mRNA microarray, immunohistochemistry assay, qRT-PCR, and western blotting to measure the expression of Wnt8b in myobroblast differentiation of LR-MSCs and BLM-induced mouse brotic lungs. Immunouorescence staining and western blotting were performed to analyze myobroblast differentiation of LR-MSCs after overexpressing or silence Wnt8b. -

WNT3A Promotes Neuronal Regeneration Upon Traumatic Brain Injury
International Journal of Molecular Sciences Article WNT3A Promotes Neuronal Regeneration upon Traumatic Brain Injury 1, 1, 2 1 1 Chu-Yuan Chang y, Min-Zong Liang y, Ching-Chih Wu , Pei-Yuan Huang , Hong-I Chen , Shaw-Fang Yet 3, Jin-Wu Tsai 4, Cheng-Fu Kao 5,* and Linyi Chen 1,6,* 1 Institute of Molecular Medicine, National Tsing Hua University, Hsinchu 30013, Taiwan; [email protected] (C.-Y.C.); [email protected] (M.-Z.L.); [email protected] (P.-Y.H.); [email protected] (H.-I.C.) 2 Department of Life Science, National Tsing Hua University, Hsinchu 30013, Taiwan; [email protected] 3 Institute of Cellular and System Medicine, National Health Research Institutes, Zhunan 35053, Taiwan; [email protected] 4 Institute of Brain Science, National Yang-Ming University, Taipei 11221, Taiwan; [email protected] 5 Institute of Cellular and Organismic Biology, Academia Sinica, Taipei 11574, Taiwan 6 Department of Medical Science, National Tsing Hua University, Hsinchu 30013, Taiwan * Correspondence: [email protected] (C.-F.K.); [email protected] (L.C.); Tel.: +886-3-574-2775 (L.C.); Fax: +886-3-571-5934 (L.C.) These authors contributed equally to this work. y Received: 20 January 2020; Accepted: 19 February 2020; Published: 21 February 2020 Abstract: The treatment of traumatic brain injury (TBI) remains a challenge due to limited knowledge about the mechanisms underlying neuronal regeneration. This current study compared the expression of WNT genes during regeneration of injured cortical neurons. Recombinant WNT3A showed positive effect in promoting neuronal regeneration via in vitro, ex vivo, and in vivo TBI models.