Why Decussate? - Topological Constraints on 3D Wiring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
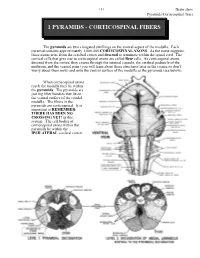
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Quantifying Nerve Decussation Abnormalities in the Optic Chiasm T Robert J
NeuroImage: Clinical 24 (2019) 102055 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Quantifying nerve decussation abnormalities in the optic chiasm T Robert J. Puzniaka, Khazar Ahmadia, Jörn Kaufmannb, Andre Gouwsc, Antony B. Morlandc,d, ⁎ Franco Pestillie,1, Michael B. Hoffmanna,f,1, a Department of Ophthalmology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany b Department of Neurology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany c York Neuroimaging Centre, Department of Psychology, University of York, York, United Kingdom d York Biomedical Research Institute, University of York, York, United Kingdom e Department of Psychological and Brain Sciences, Program in Neuroscience and Program in Cognitive Science, Indiana University, Bloomington, USA f Center for Behavioral Brain Sciences, Magdeburg, Germany ARTICLE INFO ABSTRACT Keywords: Objective: The human optic chiasm comprises partially crossing optic nerve fibers. Here we used diffusion MRI Optic chiasm (dMRI) for the in-vivo identification of the abnormally high proportion of crossing fibers found intheoptic Albinism chiasm of people with albinism. Diffusion MRI Methods: In 9 individuals with albinism and 8 controls high-resolution 3T dMRI data was acquired and analyzed Functional MRI with a set of methods for signal modeling [Diffusion Tensor (DT) and Constrained Spherical Deconvolution Crossing nerves (CSD)], tractography, and streamline filtering (LiFE, COMMIT, and SIFT2). The number of crossing andnon- crossing streamlines and their weights after filtering entered ROC-analyses to compare the discriminative power of the methods based on the area under the curve (AUC). The dMRI results were cross-validated with fMRI estimates of misrouting in a subset of 6 albinotic individuals. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

Developmental Determinants at the Mammalian Optic Chiasm
- Feature Article Developmental Determinants at the Mammalian Optic Chiasm Ft. W. Guillery,’ C. A. Mason,* and J. S. H. Taylor’ ‘Department of Human Anatomy, University of Oxford, Oxford OX1 3QX, United Kingdom and *Departments of Pathology, Anatomy, and Cell Biology, Centre for Neurobiology and Behaviour, College of Physicians and Surgeons, Columbia University, New York 10032 The optic chiasm is the point at the ventral midline of the di- ber of mutants are known that show well-defined abnormalities encephalon where the nerve fibers from the two eyes meet. Clas- of the optic chiasm. The chiasm can no longer be regarded sim- sically, it has been known as the place where groups of retinal ply as the region where some axons cross their partners from axons segregate to pass into the optic tract on either the same the other eye, and others diverge into an uncrossed course. There or the opposite side of the brain (Figs. 1, 2). This segregation is is now an opportunity to look more closely at the cellular and dependent upon the retinal position of the ganglion cells from molecular events producing the characteristic and rigidly cho- which the axons arise: axons from the nasal retina all cross to reographed patterns of axonal growth. The development of the the opposite side, whereas many from the temporal or ventro- optic chiasm and the reorganization of retinotopic order that oc- temporal part of the retina (temporal crescent) remain uncrossed. curs within region of the chiasm can now be seen in terms of a This segregation of the axons into a crossed and uncrossed com- number of discrete steps, each probably relatively simple in it- ponent allows the appropriate bilateral connections that underlie self, which together produce the final developmental sequence. -

Decussation Geometries in the Goldfish Nervous System
Proc. Natl. Acad. Sci. USA Vol. 76, No. 8, pp. 4131-4135, August 1979 Neurobiology Decussation geometries in the goldfish nervous system: Correlation with probability of survival (brain asymmetries/lateralization of function/Mauthner's neuron/optic chiasm/natural selection) RICHARD L. ROTH Department of Biological Sciences, Stanford University, Stanford, California 94305 Communicated by Donald Kennedy, May 9, 1979 ABSTRACT In the goldfish, the optic nerve decussation assumed to be representative of goldfish available in the United occurs without intermingling of fibers from the two eyes. In States. The embryonic and larval specimens examined were two-thirds of juvenile and adult specimens, the left optic nerve derived from two lots of fertile eggs and, by the breeders' es- is dorsal at the midline. In about 60% of the specimens, the decussation of Mauthner's neuron also has a left-dorsal-to-right timates, represent the progeny of 9-14 spawning females.* (L/R) configuration. Concordance for decussation geometry is Despite this limited sampling of embryos and larvae, the results greater than 80%, with smaller specimens accounting for a presented below indicate that they, too, provide an adequate disproportionate number of discordant cases. In embryos and basis for generalization. very young larvae, the L/R configuration occurs in slightly less For minimization of sampling bias, most of the Spanish moss than 50% of o tic chiasmata and in slightly more than 50% of into each Mauthner's cell chiasmata, and there is no significant tendency containing the eggs was teased and cut portions, toward concordance. However, larval specimens that survive bearing 50-75 eggs. -

Vertical Gaze Palsy and Selective Unilateral Medial Longitudinal
Journal ofNeurology, Neurosurgery, and Psychiatry 1990;53:67-71 67 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.53.1.67 on 1 January 1990. Downloaded from Vertical gaze palsy and selective unilateral infarction of the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) J Bogousslavsky, J Miklossy, F Regli, R Janzer Abstract Case report We report a clinico-pathological correla- A 60 year old housewife who was a smoker had tion study in a patient with basilar artery been investigated for ten years for arterial thrombosis, who developed tetraplegia hypertension, diabetes mellitus and elevated and combined up- and downgaze palsy blood cholesterol. Three weeks before admis- involving voluntary saccades and sion, she experienced transient dysarthria and visually-guided movements, but sparing dizziness and the day before had been unable to the oculocephalic responses. At necropsy, stand and experienced tingling in the left hand. apart from bilateral infarction in the One day later, she suffered acute rotatory basis pontis, there was a single unilateral vertigo with vomiting, diplopia, dysarthria, infarct selectively destroying the rostral and left-sided weakness and numbness. On interstitial nucleus of the medial longi- admission, the patient was well-oriented in tudinal fasciculus (riMLF) on the right. time and place but had severe dysarthria. Blood The posterior commissure and its pressure was 140/85 mm Hg. The visual fields nucleus, the nucleus of Cajal, the nucleus were normal. Eye movements were normal. of Darkschewitsch and the pontine The pupils could not be assessed, because of tegmentum were spared. We suggest that previous cataract operations. -

10 Control of Movement
Physiology Unit 2 CONTROL OF MOVEMENT In Physiology Today Motor Program • Pattern of neural activity required to properly perform the desired movement • Highest level – Motor cortex – Decide what movement will occur • Middle level – Cerebellum, basal ganglia, thalamus, brainstem, spinal cord, – Postures and movements needed to carry out the action – Receives sensory input from local level • Local level – Brainstem, Spinal cord – Afferent neurons, Motor neurons, Interneurons – Monitors muscle length and tension constantly adjusting muscle contraction Control of Body Movement Motor Control Hierarchy Local Control of Motor Neurons • Local control levels are relay points for instructions coming from higher levels in the motor program • Adjusting motor unit activity to local conditions (obstacles to movement, pain) • Local control systems use sensory information from sensory receptors – Muscles – Tendons Somatic Nervous System – Joints – Overlying skin Interneurons • Most of the synaptic input from descending pathways to motor neurons are from interneurons • 90% of spinal cord neurons • Integrate inputs – Higher centers – Peripheral receptors – Other interneurons • Determine which muscles are activated and when – Coordinates repetitive, rhythmic activities • Walking, running • Can turn movements on or off – Grabbing a hot plate Reflex Arc • The basic anatomical/functional unit of the nervous system • Components: 1. Sensory receptor 2. 1st order sensory neuron 3. Integrating center (brain/spinal cord) 4. Motor neuron 5. Effector organ (skeletal muscle, cardiac muscle, smooth muscle, glands) Reflex Arc Components 1. Sensory receptor – Constantly samples its environment • Phasic receptors • Tonic receptors 2. 1st order sensory neuron – Afferent, peripheral; leading to the CNS 3. Integrating/coordinating center – Central nervous system • Processes all incoming sensory information • Integrates sensory information • Coordinates motor commands Reflex Arc Components 4. -

Quiz 42, Understanding Motor Systemssr
M O T O R Pathways SNACC QUIZ #42 Quiz #42 UNDERSTANDING MOTOR PATHWAYS IN THE CENTRAL NERVOUS SYSTEM M. ANGELE THEARD, MD ANESTHESIOLOGIST, LEGACY EMANUEL HOSPITAL, PORTLAND OREGON QUIZ TEAM: SHOBANA RAJAN, MD SUNEETA GOLLAPUDY, MD VERGHESE CHERIAN, MD THIS QUIZ IS PUBLISHED ON BEHALF OF THE EDUCATION COMMITTEE OF SNACC To Q 1 1. MOTOR SYSTEMS IN HUMANS INCLUDE ALL OF THE FOLLOWING EXCEPT: A. Corticospinal tract B. Corticobulbar tract C. Extrapyramidal system D. Spinothalamic tract To Q 2 A. CORTICOSPINAL TRACT This is true. Axons from the corticospinal tract (CST)or pyramidal tract carry information from the precentral gyrus (brodmann area 4 of the motor cortex), the supplemental, and premotor cortices (area 6) to Lower motor neurons (LMNs) which will synapse with muscle cells in the body effecting voluntary movement. While most of the fibers from this tract are from the motor cortex, some fibers originate from the primary sensory area of the brain. Felten et al, Netter’s Atlas of Neuroscience 2nd ed. Philadelphia: Elsevier Saunders, 2010:357-86 B. CORTICOBULBAR TRACT This is true. Like the CST, axons from this descending tract originate in the motor cortex and enter the brainstem synapsing on the LMNs of cranial nerves. This tract runs alongside the CST passing through the internal capsule and into the medulla oblongata (also called bulbar) before synapsing with the LMNs of cranial nerves (CN). The muscles of the face, head and neck are controlled by the corticobulbar system. Felten et al, Netter’s Atlas of Neuroscience 2nd ed. Philadelphia: Elsevier Saunders, 2010:357-86 Liebman, et al. -

Neuroanatomy Syllabus
NEUROANATOMY AN NEUR COURSE CONTENT A T COMPETENCIES OMY The first year medical student should be able to understand and describe the gross O anatomy of central & peripheral nervous systems and correlate anatomical basis of clinical manifestations. NERVOUS TISSUE Nerve cell types, neuroglia: types, functions, blood brain barrier Level 2: Specific neuronal and neuroglial types with function Level 3: Neurotransmitters Functional components: Enumeration Afferent / Efferent; Somatic / Visceral / Branchial; General / Special Level 2: Equation with spinal and cranial nerves Level 3: Neurobiotaxis DIVISIONS OF THE NERVOUS SYSTEM: MAJOR DIVISIONS Level 2: Detailed division Level 3: Embryological link RECEPTORS AND EFFECTORS: Functional and anatomical classification; Dermatomes, myotomes Level 2: Details of functions, microanatomy, neurotransmitters, Segmental awareness Level 3: Special sense receptors (rods, cones, statoacoustic, taste buds), Axial lines, Neuromuscular junctions, muscle spindles, reflex arc SPINAL CORD Gross features: Extent (child / adult), enlargements, conus medullaris, filum terminale, spinal meninges Level 2: Spinal segments, vertebral correlation, significance of enlargements Level 3: Development, comparison with other parts of CNS, anomalies Cross sections above / below T6: TS draw and label, differences above and below T6, arrangement of grey and white matter at different levels Level 2: Lamination, nuclei of grey matter at upper & lower cervical, mid-thoracic, Lumbar & sacral levels Level 3: Details of lamination, nuclei -

Comparative Analysis of Fatty Acid Amide
Neuroscience 119 (2003) 481–496 COMPARATIVE ANALYSIS OF FATTY ACID AMIDE HYDROLASE AND CB1 CANNABINOID RECEPTOR EXPRESSION IN THE MOUSE BRAIN: EVIDENCE OF A WIDESPREAD ROLE FOR FATTY ACID AMIDE HYDROLASE IN REGULATION OF ENDOCANNABINOID SIGNALING M. EGERTOVA,´ a B. F. CRAVATTb AND M. R. ELPHICKa* throughout the mouse brain, using FAAH؊/؊ mice as negative aSchool of Biological Sciences, Queen Mary, University of London, controls to validate the specificity of FAAH-immunoreactivity London E1 4NS, UK observed in wild type animals. In many regions of the brain, a complementary pattern of FAAH and CB expression was b 1 The Skaggs Institute for Chemical Biology and Departments of Cell observed, with FAAH-immunoreactive neuronal somata and Biology and Chemistry, The Scripps Research Institute, La Jolla, CA dendrites surrounded by CB1-immunoreactive fibers. In 92037, USA these regions of the brain, FAAH may regulate postsynaptic formation of anandamide, thereby influencing the spatiotem- poral dynamics of retrograde endocannabinoid signaling. Abstract—Fatty acid amide hydrolase (FAAH) catalyses hy- However, in some regions of the brain such as the globus drolysis of the endocannabinoid arachidonoylethanolamide pallidus and substantia nigra pars reticulata, CB receptors (“anandamide”) in vitro and regulates anandamide levels in 1 are abundant but with little or no associated FAAH expres- the brain. In the cerebellar cortex, hippocampus and neocor- sion and in these brain regions the spatial impact and/or tex of the rat brain, FAAH is located in the somata and duration of endocannabinoid signaling may be less restricted dendrites of neurons that are postsynaptic to axon fibers than in regions enriched with FAAH.