BSCI338N: Diseases of the Nervous System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: a Steered-Glutamatergic Perspective
brain sciences Review Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: A Steered-Glutamatergic Perspective Amjad H. Bazzari * and H. Rheinallt Parri School of Life and Health Sciences, Aston University, Birmingham B4 7ET, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-(0)1212044186 Received: 7 October 2019; Accepted: 29 October 2019; Published: 31 October 2019 Abstract: The molecular pathways underlying the induction and maintenance of long-term synaptic plasticity have been extensively investigated revealing various mechanisms by which neurons control their synaptic strength. The dynamic nature of neuronal connections combined with plasticity-mediated long-lasting structural and functional alterations provide valuable insights into neuronal encoding processes as molecular substrates of not only learning and memory but potentially other sensory, motor and behavioural functions that reflect previous experience. However, one key element receiving little attention in the study of synaptic plasticity is the role of neuromodulators, which are known to orchestrate neuronal activity on brain-wide, network and synaptic scales. We aim to review current evidence on the mechanisms by which certain modulators, namely dopamine, acetylcholine, noradrenaline and serotonin, control synaptic plasticity induction through corresponding metabotropic receptors in a pathway-specific manner. Lastly, we propose that neuromodulators control plasticity outcomes through steering glutamatergic transmission, thereby gating its induction and maintenance. Keywords: neuromodulators; synaptic plasticity; learning; memory; LTP; LTD; GPCR; astrocytes 1. Introduction A huge emphasis has been put into discovering the molecular pathways that govern synaptic plasticity induction since it was first discovered [1], which markedly improved our understanding of the functional aspects of plasticity while introducing a surprisingly tremendous complexity due to numerous mechanisms involved despite sharing common “glutamatergic” mediators [2]. -
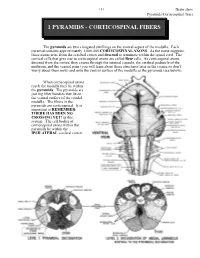
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

The Brain Stem Medulla Oblongata
Chapter 14 The Brain Stem Medulla Oblongata Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Central sulcus Parietal lobe • embryonic myelencephalon becomes Cingulate gyrus leaves medulla oblongata Corpus callosum Parieto–occipital sulcus Frontal lobe Occipital lobe • begins at foramen magnum of the skull Thalamus Habenula Anterior Epithalamus commissure Pineal gland • extends for about 3 cm rostrally and ends Hypothalamus Posterior commissure at a groove between the medulla and Optic chiasm Mammillary body pons Cerebral aqueduct Pituitary gland Fourth ventricle Temporal lobe • slightly wider than spinal cord Cerebellum Midbrain • pyramids – pair of external ridges on Pons Medulla anterior surface oblongata – resembles side-by-side baseball bats (a) • olive – a prominent bulge lateral to each pyramid • posteriorly, gracile and cuneate fasciculi of the spinal cord continue as two pair of ridges on the medulla • all nerve fibers connecting the brain to the spinal cord pass through the medulla • four pairs of cranial nerves begin or end in medulla - IX, X, XI, XII Medulla Oblongata Associated Functions • cardiac center – adjusts rate and force of heart • vasomotor center – adjusts blood vessel diameter • respiratory centers – control rate and depth of breathing • reflex centers – for coughing, sneezing, gagging, swallowing, vomiting, salivation, sweating, movements of tongue and head Medulla Oblongata Nucleus of hypoglossal nerve Fourth ventricle Gracile nucleus Nucleus of Cuneate nucleus vagus -

Electrophysiological and Morphological Evidence for a Gabaergic Nigrostriatal Pathway
The Journal of Neuroscience, June 1, 1999, 19(11):4682–4694 Electrophysiological and Morphological Evidence for a GABAergic Nigrostriatal Pathway Manuel Rodrı´guez1 and Toma´ s Gonza´ lez-Herna´ ndez2 Departments of 1Physiology and 2Anatomy, Faculty of Medicine, University of La Laguna, La Laguna, Tenerife, Canary Islands, Spain The electrophysiological and neurochemical characteristics of gic neurons, a percentage that reached 81–84% after 6-OHDA the nondopaminergic nigrostriatal (NO-DA) cells and their func- injection. Electrophysiologically, NO-DA cells showed a behavior tional response to the degeneration of dopaminergic nigrostri- similar to that found in other nigral GABAergic (nigrothalamic) atal (DA) cells were studied. Three different criteria were used to cells. In addition, the 6-OHDA degeneration of DA cells induced a identify NO-DA cells: (1) antidromic response to striatal stimu- modification of their electrophysiological pattern similar to that lation with an electrophysiological behavior (firing rate, inter- found in GABAergic nigrothalamic neurons. Taken together, the spike interval variability, and conduction velocity) different from present data indicate the existence of a small GABAergic nigro- that of DA cells; (2) retrograde labeling after striatal injection of striatal pathway and suggest their involvement in the pathophys- HRP but showing immunonegativity for DA cell markers (ty- iology of Parkinson’s disease. rosine hydroxylase, calretinin, calbindin-D28k, and cholecysto- kinin); and (3) resistance to neurotoxic effect of 6-hydroxydomine Key words: nigrostriatal pathway; dopaminergic cells; (6-OHDA). Our results showed that under normal conditions, GABAergic cells; GABA; glutamic acid decarboxylase; parval- 5–8% of nigrostriatal neurons are immunoreactive for GABA, glu- bumin; calretinin; calbindin-D28k; cholecystokinin; Parkinson’s tamic acid decarboxylase, and parvalbumin, markers of GABAer- disease The substantia nigra (SN) is a major output center of the basal paminergic (Grace and Bunney, 1980, 1983a). -

Optogenetic Activation of Dopamine Neurons in the Ventral Tegmental Area Induces Reanimation from General Anesthesia
CORE Metadata, citation and similar papers at core.ac.uk Provided by DSpace@MIT Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia Norman E. Taylora,b,c, Christa J. Van Dorta,b,c, Jonathan D. Kennya,c, JunZhu Peia,c, Jennifer A. Guideraa,c, Ksenia Y. Vlasova,c, Justin T. Leea,c, Edward S. Boydenc,d,e,f, Emery N. Browna,b,c,g,h,1,2, and Ken Solta,b,c,1 aDepartment of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA 02114; bDepartment of Anaesthesia, Harvard Medical School, Boston, MA 02114; cDepartment of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139; dMedia Lab, Massachusetts Institute of Technology, Cambridge, MA 02139; eMcGovern Institute, Massachusetts Institute of Technology, Cambridge, MA 02139; fDepartment of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139; gThe Picower Institute for Learning and Memory, Massachusetts Institute of Technology, Cambridge, MA 02139; and hInstitute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA 02139 Contributed by Emery N. Brown, September 14, 2016 (sent for review February 29, 2016; reviewed by Loren M. Frank, Robert W. Gereau, and Andrew Jenkins) Dopamine (DA) promotes wakefulness, and DA transporter inhibi- There are currently no clinically approved pharmacologic or cir- tors such as dextroamphetamine and methylphenidate are effective cuit-level interventions that can induce active emergence or for increasing arousal and inducing reanimation, or active emer- reanimation from general anesthesia (10). Dopamine (DA) is a gence from general anesthesia. DA neurons in the ventral tegmental neurotransmitter that is well-known to elicit wakefulness (11). -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Neuromodulation Shapes Interneuron Communication in the Mouse Striatum
From DEPARTMENT OF NEUROSCIENCE Karolinska Institutet, Stockholm, Sweden NEUROMODULATION SHAPES INTERNEURON COMMUNICATION IN THE MOUSE STRIATUM Matthijs Constantijn Dorst Stockholm 2020 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by US-AB © Matthijs Constantijn Dorst, 2020 ISBN 978-91-7831-908-4 Neuromodulation shapes interneuron communication in the mouse Striatum THESIS FOR DOCTORAL DEGREE (Ph.D.) By Matthijs Constantijn Dorst Principal Supervisor: Opponent: Professor Gilad Silberberg Professor Hagai Bergman Karolinska Institutet The Hebrew University of Jerusalem Department of Neuroscience Edmond & Lily Safra Center for Brain Sciences Co-supervisor(s): Examination Board: Professor Per Uhlén Professor Per Svenningsson Karolinska Institutet Karolinska Institutet Department of Medical Biochemistry and Department of Clinical Neuroscience Biophysics Division of Neuropharmacology - movement disorders Senior lecturer Karima Chergui Karolinska Institutet Department of Physiology and Pharmacology Division of Molecular Neurophysiology Professor Klas Kullander Uppsala Universitet Department of Neuroscience Research group Formation and Function of Neuronal Circuits Included Studies The following studies are included in this thesis, and will be referenced through- out the text as such: Study 1 Garas, F.N., Shah, R.S., Kormann, E., Doig, N.M., Vinciati, F., Nakamura, K.C., Dorst, M.C., Smith, Y., Magill, P.J. and Sharott, A., 2016. Sec- retagogin expression delineates functionally-specialized populations of striatal parvalbumin-containing interneurons. Elife, 5, p.e16088. Study 2 Lindroos, R., Dorst, M.C., Du, K., Filipović, M., Keller, D., Ketzef, M., Kozlov, A.K., Kumar, A., Lindahl, M., Nair, A.G., Pérez-Fernández, J., Grillner, S., Silberberg, G., Kotaleski, J.H., 2018. Basal Ganglia Neuromodulation Over Multiple Temporal and Structural Scales—Simulations of Direct Pathway MSNs Investigate the Fast Onset of Dopaminergic Effects and Predict the Role of Kv4. -

Dopaminergic Microtransplants Into the Substantia Nigra of Neonatal Rats with Bilateral 6-OHDA Lesions
The Journal of Neuroscience, May 1995, 15(5): 3548-3561 Dopaminergic Microtransplants into the Substantia Nigra of Neonatal Rats with Bilateral 6-OHDA Lesions. I. Evidence for Anatomical Reconstruction of the Nigrostriatal Pathway Guido Nikkhah,1,2 Miles G. Cunningham,3 Maria A. Cenci,’ Ronald D. McKay,4 and Anders Bj6rklund’ ‘Department of Medical Cell Research, University of Lund, S-223 62 Lund, Sweden, *Neurosurgical Clinic, Nordstadt Hospital, D-301 67 Hannover, Germany, 3Harvard Medical School, Boston, Massachusetts 02115, and 4 Laboratory of Molecular Biology, NINDS NIH, Bethesda, Maryland 20892 Reconstruction of the nigrostriatal pathway by long axon [Key words: target reinnervation, axon growth, neural growth derived from dopamine-rich ventral mesencephalic transplantation, tyrosine hydroxylase immunohistochem- (VM) transplants grafted into the substantia nigra may en- istry, Fos protein, Fluoro-Gold] hance their functional integration as compared to VM grafts implanted ectopically into the striatum. Here we report on In the lesioned brain of adult recipients dopamine-rich grafts a novel approach by which fetal VM grafts are implanted from fetal ventral mesencephalon (VM) are unable to reinner- unilaterally into the substantia nigra (SN) of 6-hydroxydo- vate the caudate-putamen unless they are placed close to, or pamine (SOHDA)-lesioned neonatal pups at postnatal day within, the denervated target structure (BjGrklund et al., 1983b; 3 (P3) using a microtransplantation technique. The results Nikkhah et al., 1994b). The failure of regenerating dopaminergic demonstrate that homotopically placed dopaminergic neu- axons to reinnervate the striatum from more distant implantation rons survive and integrate well into the previously sites, including their normal site of origin, the substantia nigra 6-OHDA-lesioned neonatal SN region. -

Brainstem and Its Associated Cranial Nerves
Brainstem and its Associated Cranial Nerves Anatomical and Physiological Review By Sara Alenezy With appreciation to Noura AlTawil’s significant efforts Midbrain (Mesencephalon) External Anatomy of Midbrain 1. Crus Cerebri (Also known as Basis Pedunculi or Cerebral Peduncles): Large column of descending “Upper Motor Neuron” fibers that is responsible for movement coordination, which are: a. Frontopontine fibers b. Corticospinal fibers Ventral Surface c. Corticobulbar fibers d. Temporo-pontine fibers 2. Interpeduncular Fossa: Separates the Crus Cerebri from the middle. 3. Nerve: 3rd Cranial Nerve (Oculomotor) emerges from the Interpeduncular fossa. 1. Superior Colliculus: Involved with visual reflexes. Dorsal Surface 2. Inferior Colliculus: Involved with auditory reflexes. 3. Nerve: 4th Cranial Nerve (Trochlear) emerges caudally to the Inferior Colliculus after decussating in the superior medullary velum. Internal Anatomy of Midbrain 1. Superior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). a. Tectospinal Pathway: turning the head, neck and eyeballs in response to a visual stimuli.1 Level of b. Spinotectal Pathway: turning the head, neck and eyeballs in response to a cutaneous stimuli.2 Superior 2. Oculomotor Nucleus: Situated in the periaqueductal grey matter. Colliculus 3. Red Nucleus: Red mass3 of grey matter situated centrally in the Tegmentum. Involved in motor control (Rubrospinal Tract). 1. Inferior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). Tectospinal Pathway: turning the head, neck and eyeballs in response to a auditory stimuli. 2. Trochlear Nucleus: Situated in the periaqueductal grey matter. Level of Inferior 3.