Direct in Vivo MRI Discrimination of Brain Stem Nuclei and Pathways
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Section of Neurosurgery, Department of Surgery, University of Michigan Hospital, Ann Arbor, Michigan
ANATOMIC PATHWAYS RELATED TO PAIN IN FACE AND NECK* JAMES A. TAREN, M.D., AND EDGAR A. KAHN, M.D. Section of Neurosurgery, Department of Surgery, University of Michigan Hospital, Ann Arbor, Michigan (Received for publication May 19, 1961) HE remarkable ability of the higher V is most dorsal in the tract. Fig. 4 is a dia- portions of the central nervous system gram of the medulla 6 mm. below the obex T to readjust to surgical lesions presup- which we believe to be the optimal level for poses the existence of alternate pathways. tractotomy. The operation is done with the This hypothesis has been tested with regard patient in the sitting position to facilitate to a specific localized sensory input, pain exposure. The medullary incision, 4-5 mm. from the face. in depth, extends from the bulbar accessory Our method has been to study the degen- rootlet to a line extrapolated from the pos- eration of nerve fibers by Weil and Marchi terior rootlets of the ~ud cervical nerve root. techniques following various surgical lesions An adequate incision results in complete in man and monkey. The lesions have con- analgesia of all 8 divisions as well as analgesia sisted of total and selective retrogasserian in the distribution of VII, IX, and X except rhizotomy in 3 humans and 3 monkeys, for sparing of the vermilion border of the lips medullary tractotomy in ~ humans and (Fig. 5). A degree of ataxia of the ipsilateral monkeys, and extirpation of the cervical upper extremity usually accompanies effec- portion of the nucleus of the descending tract tive tractotomy and is caused by compromise of V in ~ monkeys. -

Role of Endoscopy in Management of Hydrocephalus
8 Role of Endoscopy in Management of Hydrocephalus Nasser M. F. El-Ghandour Department of Neurosurgery, Faculty of Medicine, Cairo University Egypt 1. Introduction Management of hydrocephalus is the most beneficial indication for endoscopy. To cure hydrocephalus and thereby render an individual shunt independent is a great achievement. The standard and the most commonly performed endoscopic procedure is endoscopic third ventriculostomy. However, the field of neuroendoscopy is prepared to extend itself beyond just the ventriculostomy procedure. The neuroendoscope plays other important roles in the management of hydrocephalus. Although the literature is focusing mainly on the role of endoscopic third ventriculostomy in the treatment of aqueduct stenosis, the indications for endocopy continues to increase rapidly. With increasing experience, and improved endoscopic instruments, another important role of endoscopy has been emerged in approaching intraventricular tumors which may be completely removed without microsurgical brain dissection (Gaab & Schroeder, 1998). In addition to tumor removal it is usually possible to restore obstructed cerebrospinal fluid pathways using the same approach by performing ventriculostomies, septostomies or stent implantation (Oka et al., 1994). The goal of this chapter is to present the role of endoscopy in the management of hydrocephalus. The following chapter gives a comprehensive review about the indications, outcome, complications, and advantages of endoscopy. Various procedures which can be performed endoscopically for the management of hydrocephalus will be discussed. 2. Historical perspectives Endoscopy was introduced in the neurosurgical speciality at the beginning of this century. In 1910, L’Espinasse used the cystoscope to perform the first registered endoscopic procedure. He explored the lateral ventricle and coagulated the choroid plexus in 2 infants with hydrocephalus (Walker, 2001). -
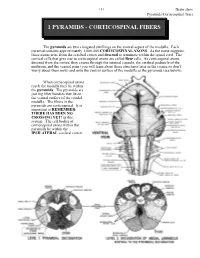
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Brainstem Exclusive of the Pyramids Themselves, Which Were Blood-Supplied Through the Intact Basilar Artery
NOTE ON CONVERGENCE OF PYRAMIDAL AND PRIMARY AFFERENT IMPULSES IN THE SPINAL CORD OF THE CAT BY DAVID P. C. LLOYD THE ROCKEFELLER UNIVERSITY Communicated December 20, 1967 The burden of this note is to present an example of observations long since made but not previously published. The immediate stimulus for presenting them now was provided by the paper by R. Porter' in which a combination of im- pulses of cortical and of lingual nerve origin was shown by convergence to facilitate the response of interneurons in or near the spinal trigeminal nucleus to a level of activity greater than that of the sum of responses elicited by lingual nerve stimu- lation and corticospinal stimulation, respectively, in isolation. Experiments of the sort now to be described concern the convergence upon interneurons in the lumbar spinal enlargement of pyramidal tract impulses and primary afferent im- pulses engendered by stimulation of the seventh lumbar dorsal root. To an ex- tent these experiments are confirmatory, with respect to another location in the neuraxis, of the observations of Porter,' but they demonstrate, in addition, how one pathway by occlusion may pre-empt the interneuron from service to the other pathway. In a prior paper2 convergence at the internuncial level was im- plied by virtue of pyramidal tract facilitatory influence upon disynaptic (three- neuron-arc) reflexes in the absence of any influence upon monosynaptic (two- neuron-arc) reflexes (ref. 2, Figs. 9 and 10). Preparation and procedure were discussed in the previous publication2 and need not be restated here, except to note that the pyramidal tract impulses were "pure" because the bulbar pyramid was stimulated rostral to a lesion that sev- ered the entire brainstem exclusive of the pyramids themselves, which were blood-supplied through the intact basilar artery. -

Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G
Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G. Jamieson, M.D. Weill Cornell Medicine, New York, NY The patient who presents with either acute or subacute confusion, in the absence of a clearly defined speech disorder and focality on neurological examination that would indicate an underlying mass lesion, needs to be evaluated for a multitude of neurological conditions. Many of the conditions that produce the recent onset of alteration in mental status, that ranges from mild confusion to florid delirium, may be due to infectious or inflammatory conditions that warrant acute intervention such as antimicrobial drugs, steroids or plasma exchange. However, some patients with recent onset of confusion have an underlying toxic-metabolic disorders indicating a specific diagnosis with need for appropriate treatment. The clinical presentations of some patients may indicate the diagnosis (e.g. hypoglycemia, chronic alcoholism) while the imaging patterns must be recognized to make the diagnosis in other patients. Toxic-metabolic disorders constitute a group of diseases and syndromes with diverse causes and clinical presentations. Many toxic-metabolic disorders have no specific neuroimaging correlates, either at early clinical stages or when florid symptoms develop. However, some toxic-metabolic disorders have characteristic abnormalities on neuroimaging, as certain areas of the central nervous system appear particularly vulnerable to specific toxins and metabolic perturbations. Areas of particular vulnerability in the brain include: 1) areas of high-oxygen demand (e.g. basal ganglia, cerebellum, hippocampus), 2) the cerebral white matter and 3) the mid-brain. Brain areas of high-oxygen demand are particularly vulnerable to toxins that interfere with cellular respiratory metabolism. -

DR. Sanaa Alshaarawy
By DR. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific criteria of each level. 1. Medulla oblongata (closed, mid and open medulla) 2. Pons (caudal, mid “Trigeminal level” and rostral). 3. Mid brain ( superior and inferior colliculi). Describe the Reticular formation (structure, function and pathway) being an important content of the brain stem. 2 1. Traversed by the Central Canal. Motor Decussation*. Spinal Nucleus of Trigeminal (Trigeminal sensory nucleus)* : ➢ It is a larger sensory T.S of Caudal part of M.O. nucleus. ➢ It is the brain stem continuation of the Substantia Gelatinosa of spinal cord 3 The Nucleus Extends : Through the whole length of the brain stem and upper segments of spinal cord. It lies in all levels of M.O, medial to the spinal tract of the trigeminal. It receives pain and temperature from face, forehead. Its tract present in all levels of M.O. is formed of descending fibers that terminate in the trigeminal nucleus. 4 It is Motor Decussation. Formed by pyramidal fibers, (75-90%) cross to the opposite side They descend in the Decuss- = crossing lateral white column of the spinal cord as the lateral corticospinal tract. The uncrossed fibers form the ventral corticospinal tract. 5 Traversed by Central Canal. Larger size Gracile & Cuneate nuclei, concerned with proprioceptive deep sensations of the body. Axons of Gracile & Cuneate nuclei form the internal arcuate fibers; decussating forming Sensory Decussation. Pyramids are prominent ventrally. 6 Formed by the crossed internal arcuate fibers Medial Leminiscus: Composed of the ascending internal arcuate fibers after their crossing. -

An EM Study of the Dorsal Nucleus of the Lateral Lemniscus: Inhibitory, Commissural, Synaptic Connections Between Ascending Auditory Pathways
The Journal of Neuroscience, March 1989, g(3): 987-982 An EM Study of the Dorsal Nucleus of the Lateral Lemniscus: Inhibitory, Commissural, Synaptic Connections Between Ascending Auditory Pathways Douglas L. Oliver and Amiram Shneiderman Department of Anatomy, The University of Connecticut Health Center, Farmington, Connecticut 06032 The dorsal nucleus of the lateral lemniscus (DNLL) and its tions between the nuclei. Crossed inhibitory connections in connections constitute one of the ascending auditory path- the DNLL pathway may be important in regulating the flow ways to the inferior colliculus. One notable feature of this of ascending auditory information. nucleus is the heavy commissural connections between DNLL on opposite sides of the midbrain. These commissural con- The dorsal nucleus of the lateral lemniscus (DNLL) holds a nections may have a significant impact on the ascending unique position in the auditory system. Astride the fibers of the pathway. In this study, the fine structure of DNLL in the cat lateral lemniscus,DNLL receives most of the sameprojections and its commissural connections were examined. Both an- that ascendto the inferior colliculus (Glendenning et al., 1981; terograde and retrograde transport methods were used si- Shneiderman et al., 1988); and DNLL also projects bilaterally multaneously at the EM level. Injections of 3H-leucine mixed to the inferior colliculus. Thus, the combined inputs and outputs with WGA-HRP were made in one DNLL. After axonal trans- of DNLL form a multisynaptic pathway, parallel to the other port, EM autoradiographic methods were used to identify the ascending pathways to the inferior colliculus (Goldberg and anterogradely labeled axonal endings from the opposite Moore, 1967; van Noort, 1969; Roth et al., 1978; Adams, 1979; DNLL. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Neural Plasticity in the Brain During Neuropathic Pain
biomedicines Review Neural Plasticity in the Brain during Neuropathic Pain Myeong Seong Bak 1, Haney Park 1 and Sun Kwang Kim 1,2,* 1 Department of Science in Korean Medicine, Graduate School, Kyung Hee University, Seoul 02447, Korea; [email protected] (M.S.B.); [email protected] (H.P.) 2 Department of Physiology, College of Korean Medicine, Kyung Hee University, Seoul 02447, Korea * Correspondence: [email protected]; Tel.: +82-2-961-0491 Abstract: Neuropathic pain is an intractable chronic pain, caused by damage to the somatosensory nervous system. To date, treatment for neuropathic pain has limited effects. For the development of efficient therapeutic methods, it is essential to fully understand the pathological mechanisms of neuropathic pain. Besides abnormal sensitization in the periphery and spinal cord, accumulating evidence suggests that neural plasticity in the brain is also critical for the development and mainte- nance of this pain. Recent technological advances in the measurement and manipulation of neuronal activity allow us to understand maladaptive plastic changes in the brain during neuropathic pain more precisely and modulate brain activity to reverse pain states at the preclinical and clinical levels. In this review paper, we discuss the current understanding of pathological neural plasticity in the four pain-related brain areas: the primary somatosensory cortex, the anterior cingulate cortex, the periaqueductal gray, and the basal ganglia. We also discuss potential treatments for neuropathic pain based on the modulation of neural plasticity in these brain areas. Keywords: neuropathic pain; neural plasticity; primary somatosensory cortex; anterior cingulate cortex; periaqueductal grey; basal ganglia Citation: Bak, M.S.; Park, H.; Kim, S.K.