Internuclear Ophthalmoplegia: MR-Anatomic Correlation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Magnetic Resonance Imaging of Multiple Sclerosis: a Study of Pulse-Technique Efficacy
691 Magnetic Resonance Imaging of Multiple Sclerosis: A Study of Pulse-Technique Efficacy Val M. Runge1 Forty-two patients with the clinical diagnosis of multiple sclerosis were examined by Ann C. Price1 proton magnetic resonance imaging (MRI) at 0.5 T. An extensive protocol was used to Howard S. Kirshner2 facilitate a comparison of the efficacy of different pulse techniques. Results were also Joseph H. Allen 1 compared in 39 cases with high-resolution x-ray computed tomography (CT). MRI revealed characteristic abnormalities in each case, whereas CT was positive in only 15 C. Leon Partain 1 of 33 patients. Milder grades 1 and 2 disease were usually undetected by CT, and in all A. Everette James, Jr.1 cases, the abnormalities noted on MRI were much more extensive than on CT. Cerebral abnormalities were best shown with the T2-weighted spin-echo sequence (TE/TR = 120/1000); brainstem lesions were best defined on the inversion-recovery sequence (TE/TI/TR =30/400/1250). Increasing TE to 120 msec and TR to 2000 msec heightened the contrast between normal and abnormal white matter. However, the signal intensity of cerebrospinal fluid with this pulse technique obscured some abnormalities. The diagnosis of multiple sclerosis continues to be a clinical challenge [1,2). The lack of an objective means of assessment further complicates the evaluation of treatment regimens. Evoked potentials, cerebrospinal fluid (CSF) analysis , and computed tomography (CT) are currently used for diagnosis, but all lack sensitivity and/or specificity. Furthermore, postmortem examinations demonstrate many more lesions than those suggested by clinical means [3). -
Corticospinal Fibers
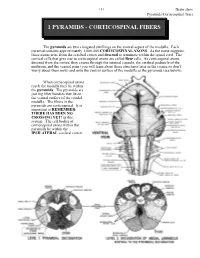
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Brainstem: Structure & Its Mode of Action
Journal of Neurology & Neurophysiology 2021, Vol.12, Issue 3, 521 Opinion Brainstem: Structure & Its Mode of action Karthikeyan Rupani Research Fellow, Tata Medical Centre, India. Corresponding Author* The brainstem is exceptionally little, making up around as it were 2.6 percent of the brain's add up to weight. It has the basic parts of directing cardiac, and Rupani K, respiratory work, making a difference to control heart rate and breathing rate. Research Fellow, Tata Medical Centre, India; It moreover gives the most engine and tactile nerve supply to the confront and E-mail: [email protected] neck by means of the cranial nerves. Ten sets of cranial nerves come from the brainstem. Other parts incorporate the direction of the central apprehensive Copyright: 2021 Rupani K. This is an open-access article distributed under the framework and the body's sleep cycle. It is additionally of prime significance terms of the Creative Commons Attribution License, which permits unrestricted within the movement of engine and tangible pathways from the rest of the use, distribution, and reproduction in any medium, provided the original author brain to the body, and from the body back to the brain. These pathways and source are credited. incorporate the corticospinal tract (engine work), the dorsal column-medial lemniscus pathway and the spinothalamic tract [3]. The primary part of the brainstem we'll consider is the midbrain. The midbrain Received 01 March 2021; Accepted 15 March 2021; Published 22 March 2021 (too known as the mesencephalon) is the foremost prevalent of the three districts of the brainstem. It acts as a conduit between the forebrain over and the pons and cerebellum underneath. -

Sylvian Aqueduct Syndrome and Global Rostral Midbrain Dysfunction Associated with Shunt Malfunction
Sylvian aqueduct syndrome and global rostral midbrain dysfunction associated with shunt malfunction Giuseppe Cinalli, M.D., Christian Sainte-Rose, M.D., Isabelle Simon, M.D., Guillaume Lot, M.D., and Spiros Sgouros, M.D. Department of Pediatric Neurosurgery and Pediatric Radiology, Hôpital Necker•Enfants Malades, Université René Decartes; and Department of Neurosurgery, Hôpital Lariboisiere, Paris, France Object. This study is a retrospective analysis of clinical data obtained in 28 patients affected by obstructive hydrocephalus who presented with signs of midbrain dysfunction during episodes of shunt malfunction. Methods. All patients presented with an upward gaze palsy, sometimes associated with other signs of oculomotor dysfunction. In seven cases the ocular signs remained isolated and resolved rapidly after shunt revision. In 21 cases the ocular signs were variably associated with other clinical manifestations such as pyramidal and extrapyramidal deficits, memory disturbances, mutism, or alterations in consciousness. Resolution of these symptoms after shunt revision was usually slow. In four cases a transient paradoxical aggravation was observed at the time of shunt revision. In 11 cases ventriculocisternostomy allowed resolution of the symptoms and withdrawal of the shunt. Simultaneous supratentorial and infratentorial intracranial pressure recordings performed in seven of the patients showed a pressure gradient between the supratentorial and infratentorial compartments with a higher supratentorial pressure before shunt revision. Inversion of this pressure gradient was observed after shunt revision and resolution of the gradient was observed in one case after third ventriculostomy. In six recent cases, a focal midbrain hyperintensity was evidenced on T2-weighted magnetic resonance imaging sequences at the time of shunt malfunction. This rapidly resolved after the patient underwent third ventriculostomy. -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens)
Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens) Lecture (13) ▪ Important ▪ Doctors Notes Please check our Editing File ▪ Notes/Extra explanation ه هذا العمل مب ين بشكل أسا يس عىل عمل دفعة 436 مع المراجعة { َوَم نْ يَ َت َو َ ّكْ عَ َلْ ا َّْلل فَهُ َوْ َحْ سْ ُ ُُْ} والتدقيق وإضافة المﻻحظات وﻻ يغ ين عن المصدر اﻷسا يس للمذاكرة ▪ Objectives At the end of the lecture, students should be able to: ✓ List the cranial nuclei related to occulomotor, trochlear, and abducent nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the site of emergence and course of these 3 nerves. ✓ Describe the important relations of oculomotor, trochlear, and abducent nerves in the orbit ✓ List the orbital muscles supplied by each of these 3 nerves. ✓ Describe the effect of lesion of each of these 3 nerves. ✓ Describe the optic nerve and visual pathway. Recall the how these nerves exit from the brain stem: Optic (does not exit from brain stem) Occulomotor: ventral midbrain (medial aspect of crus cerebri) Trochlear: dorsal midbrain (caudal to inferior colliculus) Abducent: ventral Pons (junction b/w pons & pyramid) Brain (Ventral view) Brain stem (Lateral view) Extra-Ocular Muscles 7 muscles: (ترفع جفن العين) .Levator palpebrae superioris 1- Origin: from the roof of the orbit (4) Recti muscles: *Rectus: ماشي على ( Superior rectus (upward and medially 2- الصراط (Inferior rectus (downward and medially 3- المستقيم 4- Medial rectus (medial) (medial) 5- Lateral rectus (lateral) How to remember the 2 فحركته muscles not supplied by نفس اسمه -اسمها عكس وظيفتها- :Oblique muscles (2) 6- Superior oblique (downward and laterally) Oblique: CN3? Superior oblique goes -1 منحرفOrigin: from the roof of the orbit 7- Inferior oblique (upward and laterally) up (superior) and turns around (oblique) a notch يمشي Origin: from the anterior floor or pulley and its supply is عكس كﻻمه NB. -

Orienting Head Movements Resulting from Electrical Microstimulation of the Brainstem Tegmentum in the Barn Owl
The Journal of Neuroscience, January 1993, 13(l): 351370 Orienting Head Movements Resulting from Electrical Microstimulation of the Brainstem Tegmentum in the Barn Owl Tom Masino and Eric I. Knudsen Department of Neurobiology, Stanford University, Stanford, California 943055401 The size and direction of orienting movements are repre- movement latency, duration, velocity, and size each dem- sented systematically as a motor map in the optic tectum of onstrated dependencies on stimulus amplitude, frequency, the barn owl (du Lac and Knudsen, 1990). The optic tectum and duration. projects to several distinct regions in the medial brainstem The data demonstrate directly that at the level of the mid- tegmentum, which in turn project to the spinal cord (Masino brain tegmentum there exists a three-dimensional Cartesian and Knudsen, 1992). This study explores the hypothesis that representation of head-orienting movements such that hor- a fundamental transformation in the neural representation izontal, vertical, and roll components of movement are en- of orienting movements takes place in the brainstem teg- coded by anatomically distinct neural circuits. The data sug- mentum. Head movements evoked by electrical microstim- gest that in the projection from the optic tectum to these ulation in the brainstem tegmentum of the alert barn owl were medial tegmental regions, the topographic code for orienting cataloged and the sites of stimulation were reconstructed movement that originates in the tectum is transformed into histologically. Movements elicited from the brainstem teg- this Cartesian code. mentum were categorized into one of six different classes: [Key words: optic tectum, superior colliculus, saccadic saccadic head rotations, head translations, facial move- head movement, brainstem tegmentum, interstitial nucleus ments, vocalizations, limb movements, and twitches. -

Brainstem Dysfunction in Critically Ill Patients
Benghanem et al. Critical Care (2020) 24:5 https://doi.org/10.1186/s13054-019-2718-9 REVIEW Open Access Brainstem dysfunction in critically ill patients Sarah Benghanem1,2 , Aurélien Mazeraud3,4, Eric Azabou5, Vibol Chhor6, Cassia Righy Shinotsuka7,8, Jan Claassen9, Benjamin Rohaut1,9,10† and Tarek Sharshar3,4*† Abstract The brainstem conveys sensory and motor inputs between the spinal cord and the brain, and contains nuclei of the cranial nerves. It controls the sleep-wake cycle and vital functions via the ascending reticular activating system and the autonomic nuclei, respectively. Brainstem dysfunction may lead to sensory and motor deficits, cranial nerve palsies, impairment of consciousness, dysautonomia, and respiratory failure. The brainstem is prone to various primary and secondary insults, resulting in acute or chronic dysfunction. Of particular importance for characterizing brainstem dysfunction and identifying the underlying etiology are a detailed clinical examination, MRI, neurophysiologic tests such as brainstem auditory evoked potentials, and an analysis of the cerebrospinal fluid. Detection of brainstem dysfunction is challenging but of utmost importance in comatose and deeply sedated patients both to guide therapy and to support outcome prediction. In the present review, we summarize the neuroanatomy, clinical syndromes, and diagnostic techniques of critical illness-associated brainstem dysfunction for the critical care setting. Keywords: Brainstem dysfunction, Brain injured patients, Intensive care unit, Sedation, Brainstem -

Split Cerebral Aqueduct: a Neuroendoscopic Illustration
Split cerebral aqueduct: a neuroendoscopic illustration Alberto Feletti, Alessandro Fiorindi & Pierluigi Longatti Child's Nervous System ISSN 0256-7040 Volume 32 Number 1 Childs Nerv Syst (2016) 32:199-203 DOI 10.1007/s00381-015-2827-y 1 23 Your article is protected by copyright and all rights are held exclusively by Springer- Verlag Berlin Heidelberg. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Childs Nerv Syst (2016) 32:199–203 DOI 10.1007/s00381-015-2827-y CASE REPORT Split cerebral aqueduct: a neuroendoscopic illustration Alberto Feletti1 & Alessandro Fiorindi2 & Pierluigi Longatti2 Received: 30 June 2015 /Accepted: 9 July 2015 /Published online: 1 August 2015 # Springer-Verlag Berlin Heidelberg 2015 Abstract Introduction Purpose Forking of the cerebral aqueduct is a developmental malformation that is infrequently encountered by neurosur- Although some authors have described the forking of the ce- geons as a rare cause of hydrocephalus, sometimes with a rebral aqueduct (CA) as a common cause of hydrocephalus, a delayed onset. -

Isolated Necrosis of Central Tegmental Tracts Due to Neonatal Hypoxic-Ischemic Encephalopathy: MRI Findings
Journal of Neurology & Stroke Case Report Open Access Isolated necrosis of central tegmental tracts due to neonatal hypoxic-ischemic encephalopathy: MRI findings Abstract Volume 11 Issue 1 - 2021 Perinatal hypoxia is an old entity that still prevails today and may lead to neurological Tomás de Andrade Lourenção Freddi, Luiz sequelae that can go unnoticed until a certain age, generating many costs for public health. In this case report, we demonstrate on magnetic resonance imaging an unusual pattern of Fellipe Curvêlo Ciraulo Santos, Nelson Paes perinatal hypoxia in a preterm 5-month-old infant, involving the central tegmental tracts Fortes Diniz Ferreira, Felipe Diego Gomes and briefly discuss its possible pathophysiology. Dantas Department of Radiology, Hospital do Coração, Brazil Keywords: magnetic resonance imaging, asphyxia, hypoxic-ischemic encephalopathy, tegmentum, neonates, brainstem Correspondence: Tomás de Andrade Lourenção Freddi, Hospital do Coração, 147 Desembargador Eliseu Guilherme Street, São Paulo, SP, 04004-030, Brazil, Tel +5511976059280, Email Received: May 25, 2020 | Published: Febrauary 15, 2021 Abbreviations: MRI, magnetic resonance imaging; HII, that connect the red nucleus and the inferior olivary nucleus, being hypoxic-ischemic injury; FLAIR, fluid-attenuated inversion recovery; part of the dentato-rubro-olivary system, called Guillain–Mollaret CTT, central tegmental tract; VGB, vigabatrin triangle.3–5 Introduction Hypoxic-ischemic injury (HII) is one of the most important causes of encephalopathy in neonates, irrespective of gestational age, and may occur in the uterus or during delivery by different intrapartum conditions. In preterm or very low birth weight infants, brain magnetic resonance imaging (MRI) can demonstrate multiple different findings, which a detailed description is beyond the scope of this article, although being periventricular leukomalacia the most frequent (seen in at least 50% of cases). -

The Accessory Optic System: Basic Organization with an Update on Connectivity, Neurochemistry, and Function
UC Irvine UC Irvine Previously Published Works Title The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function. Permalink https://escholarship.org/uc/item/3v25z604 Journal Progress in brain research, 151 ISSN 0079-6123 Authors Giolli, Roland A Blanks, Robert H I Lui, Fausta Publication Date 2005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Chapter 13 The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function Roland A. Giolli1, , , Robert H.I. Blanks1, 2 and Fausta Lui3 1Department of Anatomy and Neurobiology, University of California, College of Medicine, Irvine, CA 92697, USA 2Charles E. Schmidt College of Science, Florida Atlantic University, 777 Glades Rd., P.O. Box 3091, Boca Raton, FL 33431, USA 3Dipartimento di Scienze Biomediche, Sezione di Fisiologia, Universita di Modena e Reggio Emilia, Via Campi 287, 41100, Modena, Italy Available online 10 October 2005. Abstract The accessory optic system (AOS) is formed by a series of terminal nuclei receiving direct visual information from the retina via one or more accessory optic tracts. In addition to the retinal input, derived from ganglion cells that characteristically have large receptive fields, are direction-selective, and have a preference for slow moving stimuli, there are now well-characterized afferent connections with a key pretectal nucleus (nucleus of the optic tract) and the ventral lateral geniculate nucleus. The efferent connections of the AOS are robust, targeting brainstem and other structures in support of visual-oculomotor events such as optokinetic nystagmus and visual–vestibular interaction. This chapter reviews the newer experimental findings while including older data concerning the structural and functional organization of the AOS.