An Ancestral Axial Twist Explains the Contralateral Forebrain and the Optic Chiasm in Vertebrates$
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
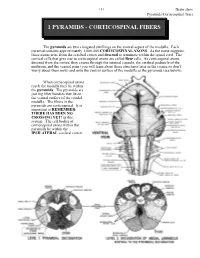
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Cranial Nerve Palsy
Cranial Nerve Palsy What is a cranial nerve? Cranial nerves are nerves that lead directly from the brain to parts of our head, face, and trunk. There are 12 pairs of cranial nerves and some are involved in special senses (sight, smell, hearing, taste, feeling) while others control muscles and glands. Which cranial nerves pertain to the eyes? The second cranial nerve is called the optic nerve. It sends visual information from the eye to the brain. The third cranial nerve is called the oculomotor nerve. It is involved with eye movement, eyelid movement, and the function of the pupil and lens inside the eye. The fourth cranial nerve is called the trochlear nerve and the sixth cranial nerve is called the abducens nerve. They each innervate an eye muscle involved in eye movement. The fifth cranial nerve is called the trigeminal nerve. It provides facial touch sensation (including sensation on the eye). What is a cranial nerve palsy? A palsy is a lack of function of a nerve. A cranial nerve palsy may cause a complete or partial weakness or paralysis of the areas served by the affected nerve. In the case of a cranial nerve that has multiple functions (such as the oculomotor nerve), it is possible for a palsy to affect all of the various functions or only some of the functions of that nerve. What are some causes of a cranial nerve palsy? A cranial nerve palsy can occur due to a variety of causes. It can be congenital (present at birth), traumatic, or due to blood vessel disease (hypertension, diabetes, strokes, aneurysms, etc). -

MR Imaging of Primary Trochlear Nerve Neoplasms
707 MR Imaging of Primary Trochlear Nerve Neoplasms Lindell R. Gentry 1 We present the clinical, anatomic, and MR imaging findings in six patients with seven Rahul C. Mehta 1 primary trochlear nerve neoplasms, as well as the MR and clinical criteria that serve to Richard E. Appen2 establish the diagnosis of these rare cranial nerve neoplasms. Three patients had a Joel M. Weinstein2 history of neurofibromatosis and five patients had clinical evidence of a trochlear nerve palsy. Six of seven neoplasms produced localized, fusiform enlargement of the proximal cisternal segments of the trochlear nerves. The lesions that were visible on noncontrast MR scans (T1-, T2-, and proton density-weighted) had signal intensities that were virtually identical to normal brain parenchyma. All lesions showed intense, homogeneous enhancement on contrast-enhanced scans. Contrast-enhanced imaging was necessary for the detection of five of seven lesions and greatly increased the value of the MR study in all six patients. AJNR 12:707-713, July/August 1991; AJR 157: September 1991 Primary neoplasms arising from pure motor cranial nerves such as the trochlear nerve are rare. To our knowledge just nine primary trochlear nerve neoplasms have been reported in the literature [1-7), only one of which was imaged with MR [1). Over the last 6 years, since we began to use MR as the primary imaging method for evaluating patients with cranial nerve palsies , we have noticed a striking increase in the number of primary trochlear nerve neoplasms over those encountered during the CT era. We believe this is due to a much greater sensitivity of MR in detecting these typically small lesions. -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Cranial Nerve Disorders: Clinical Manifestations and Topographyଝ
Radiología. 2019;61(2):99---123 www.elsevier.es/rx UPDATE IN RADIOLOGY Cranial nerve disorders: Clinical manifestations and topographyଝ a,∗ a b c M. Jorquera Moya , S. Merino Menéndez , J. Porta Etessam , J. Escribano Vera , a M. Yus Fuertes a Sección de Neurorradiología, Hospital Clínico San Carlos, Madrid, Spain b Servicio de Neurología, Hospital Clínico San Carlos, Madrid, Spain c Neurorradiología, Hospital Ruber Internacional, Madrid, Spain Received 17 November 2017; accepted 27 September 2018 KEYWORDS Abstract The detection of pathological conditions related to the twelve cranial pairs rep- Cranial pairs; resents a significant challenge for both clinicians and radiologists; imaging techniques are Cranial nerves; fundamental for the management of many patients with these conditions. In addition to knowl- Cranial neuropathies; edge about the anatomy and pathological entities that can potentially affect the cranial pairs, Neuralgia; the imaging evaluation of patients with possible cranial pair disorders requires specific exami- Cranial nerve palsy nation protocols, acquisition techniques, and image processing. This article provides a review of the most common symptoms and syndromes related with the cranial pairs that might require imaging tests, together with a brief overview of the anatomy, the most common underlying processes, and the most appropriate imaging tests for different indications. © 2018 SERAM. Published by Elsevier Espana,˜ S.L.U. All rights reserved. PALABRAS CLAVE Sintomatología derivada de los pares craneales: Clínica y topografía Pares craneales; Resumen La detección de la patología relacionada con los doce pares craneales representa Nervios craneales; un importante desafío, tanto para los clínicos como para los radiólogos. Las técnicas de imagen Neuropatía de pares craneales; son fundamentales para el manejo de muchos de los pacientes. -

Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves
Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves Paul Rea AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands 225 Wyman Street, Waltham, MA 02451, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA First published 2014 Copyright r 2014 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -

Quantifying Nerve Decussation Abnormalities in the Optic Chiasm T Robert J
NeuroImage: Clinical 24 (2019) 102055 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Quantifying nerve decussation abnormalities in the optic chiasm T Robert J. Puzniaka, Khazar Ahmadia, Jörn Kaufmannb, Andre Gouwsc, Antony B. Morlandc,d, ⁎ Franco Pestillie,1, Michael B. Hoffmanna,f,1, a Department of Ophthalmology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany b Department of Neurology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany c York Neuroimaging Centre, Department of Psychology, University of York, York, United Kingdom d York Biomedical Research Institute, University of York, York, United Kingdom e Department of Psychological and Brain Sciences, Program in Neuroscience and Program in Cognitive Science, Indiana University, Bloomington, USA f Center for Behavioral Brain Sciences, Magdeburg, Germany ARTICLE INFO ABSTRACT Keywords: Objective: The human optic chiasm comprises partially crossing optic nerve fibers. Here we used diffusion MRI Optic chiasm (dMRI) for the in-vivo identification of the abnormally high proportion of crossing fibers found intheoptic Albinism chiasm of people with albinism. Diffusion MRI Methods: In 9 individuals with albinism and 8 controls high-resolution 3T dMRI data was acquired and analyzed Functional MRI with a set of methods for signal modeling [Diffusion Tensor (DT) and Constrained Spherical Deconvolution Crossing nerves (CSD)], tractography, and streamline filtering (LiFE, COMMIT, and SIFT2). The number of crossing andnon- crossing streamlines and their weights after filtering entered ROC-analyses to compare the discriminative power of the methods based on the area under the curve (AUC). The dMRI results were cross-validated with fMRI estimates of misrouting in a subset of 6 albinotic individuals. -

Cranial Nerves
Cranial Nerves Cranial nerve evaluation is an important part of a neurologic exam. There are some differences in the assessment of cranial nerves with different species, but the general principles are the same. You should know the names and basic functions of the 12 pairs of cranial nerves. This PowerPage reviews the cranial nerves and basic brain anatomy which may be seen on the VTNE. The 12 Cranial Nerves: CN I – Olfactory Nerve • Mediates the sense of smell, observed when the pet sniffs around its environment CN II – Optic Nerve Carries visual signals from retina to occipital lobe of brain, observed as the pet tracks an object with its eyes. It also causes pupil constriction. The Menace response is the waving of the hand at the dog’s eye to see if it blinks (this nerve provides the vision; the blink is due to cranial nerve VII) CN III – Oculomotor Nerve • Provides motor to most of the extraocular muscles (dorsal, ventral, and medial rectus) and for pupil constriction o Observing pupillary constriction in PLR CN IV – Trochlear Nerve • Provides motor function to the dorsal oblique extraocular muscle and rolls globe medially © 2018 VetTechPrep.com • All rights reserved. 1 Cranial Nerves CN V – Trigeminal Nerve – Maxillary, Mandibular, and Ophthalmic Branches • Provides motor to muscles of mastication (chewing muscles) and sensory to eyelids, cornea, tongue, nasal mucosa and mouth. CN VI- Abducens Nerve • Provides motor function to the lateral rectus extraocular muscle and retractor bulbi • Examined by touching the globe and observing for retraction (also tests V for sensory) Responsible for physiologic nystagmus when turning head (also involves III, IV, and VIII) CN VII – Facial Nerve • Provides motor to muscles of facial expression (eyelids, ears, lips) and sensory to medial pinna (ear flap). -

Central Fourth Nerve Palsies Mitchell S.V
RESIDENT &FELLOW SECTION Pearls and Oy-sters: Section Editor Central fourth nerve palsies Mitchell S.V. Elkind, MD, MS Daniel R. Gold, DO CLINICAL PEARLS Lesions of the fourth (trochlear) Clinical features suggestive of bilateral fourth nerve Robert K. Shin, MD cranial nerve cause vertical or oblique diplopia by impair- palsies include right hypertropia in left gaze, left hyper- Steven Galetta, MD ing the ability of the superior oblique muscle to intort tropia in right gaze, and alternating hypertropia with and depress the eye. This binocular diplopia worsens in head tilt to either side (i.e., right hypertropia with right downgaze and lateral gaze away from the affected eye. tilt and left hypertropia with left head tilt).8 Correspondence & reprint Because intorsion is necessary to maintain fusion in ocu- requests to Dr. Gold: [email protected] lar counter-roll, this diplopia also worsens with head tilt CASE REPORTS Case 1. A20-year-oldmanpresented 1,2 toward the affected eye. to the emergency department complaining of 6 days of Diagnosis of a superior oblique palsy can be made binocular vertical diplopia and a left eyelid droop. He using the Parks-Bielschowsky 3-step test: 1) determine had noted fatigue, bilateral eye pain, and flu-like symp- which eye is hypertropic, 2) determine if the hypertro- toms 2 to 4 weeks prior to presentation. pia worsens in left or right gaze, and 3) determine if the Left-sided ptosis and miosis were present on exam- hypertropia worsens in right or left head tilt. In a supe- ination, along with a left adduction deficit. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

Cranial Nerves. Sensory Organs
Kharkiv National Medical University, Department of Human Anatomy CRANIAL NERVES. SENSORY ORGANS. The cranial nerves are: CN I - Olfactory Nerve CN II - Optic Nerve CN III - Oculomotor Nerve CN IV - Trochlear Nerve CN V - Trigeminal Nerve CN VI - Abducens Nerve CN VII - Facial Nerve CN VIII - Vestibulocochlear Nerve CN IX - Glossopharyngeal Nerve CN X - Vagus Nerve CN XI - Accessory Nerve CN XII - Hypoglossal Nerve Classification of the Cranial Nerves It is possible to describe a cranial nerve in terms of its function and embryological origin, initially cranial nerves can be subdivided into being either: Motor (efferent) Sensory (afferent) And from there further categorization can occur. Motor (efferent) Cranial nerves -Somatic motor (general somatic efferent) (III, IV, VI, XII) These cranial nerves are so called because they innervate muscles derived from the occipital somites, such as the extra ocular and extrinsic tongue muscles. Sensory (afferent) cranial nerves -Visceral sensory special visceral afferent- (VII, IX, X) general visceral afferent- (IX, X) The name is related to the fact that it detects sensation from visceral organs. They are divided into special visceral, referring to the rostral portion of the nucleus which contributes to the special sensation of taste. Whilst the general visceral portion is named as such due to this caudal portion receiving general sensory impulses such as cardiac, respiratory . - General somatic sensory (general somatic afferent) (V, VII, IX, X) These nuclei detect general sensation, such as touch, pain, vibration from the face, sinuses and meninges - Special somatic sensory (special somatic) (VIII) This carries information from the special sensation of hearing and balance.