1 Revision 2 1 Stability Field of the Cl-Rich Scapolite Marialite 2 3 Kaleo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shear Zone Initiation in the Marcy Anorthosite Massif, Adirondacks, New York, USA James Hodge University of Maine, [email protected]
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library Summer 8-23-2019 Fractures, Fluids, and Metamorphism: Shear Zone Initiation in the Marcy Anorthosite Massif, Adirondacks, New York, USA James Hodge University of Maine, [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/etd Part of the Geochemistry Commons, Geology Commons, and the Tectonics and Structure Commons Recommended Citation Hodge, James, "Fractures, Fluids, and Metamorphism: Shear Zone Initiation in the Marcy Anorthosite Massif, Adirondacks, New York, USA" (2019). Electronic Theses and Dissertations. 3050. https://digitalcommons.library.umaine.edu/etd/3050 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. FRACTURES, FLUIDS, AND METAMORPHISM: SHEAR ZONE INITIATION IN THE MARCY ANORTHOSITE MASSIF, ADIRONDACKS, NEW YORK, USA By James Hodge B.S. College of Saint Rose, 2017 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science (in Earth and Climate Sciences) The Graduate School The University of Maine August 2019 Advisory Committee: Scott Johnson, Professor and Director School of Earth and Climate Sciences, Advisor Chris Gerbi, Professor School of Earth and Climate Sciences, Advisor Alicia Cruz-Uribe, Professor School of Earth and Climate Sciences Martin Yates, Professor School of Earth and Climate Sciences FRACTURES, FLUIDS, AND METAMORPHISM: SHEAR ZONE INITIATION IN THE MARCY ANORTHOSITE MASSIF, ADIRONDACKS, NEW YORK, USA By James Hodge Thesis Advisors: Dr. -

Optical Properties of Common Rock-Forming Minerals
AppendixA __________ Optical Properties of Common Rock-Forming Minerals 325 Optical Properties of Common Rock-Forming Minerals J. B. Lyons, S. A. Morse, and R. E. Stoiber Distinguishing Characteristics Chemical XI. System and Indices Birefringence "Characteristically parallel, but Mineral Composition Best Cleavage Sign,2V and Relief and Color see Fig. 13-3. A. High Positive Relief Zircon ZrSiO. Tet. (+) 111=1.940 High biref. Small euhedral grains show (.055) parallel" extinction; may cause pleochroic haloes if enclosed in other minerals Sphene CaTiSiOs Mon. (110) (+) 30-50 13=1.895 High biref. Wedge-shaped grains; may (Titanite) to 1.935 (0.108-.135) show (110) cleavage or (100) Often or (221) parting; ZI\c=51 0; brownish in very high relief; r>v extreme. color CtJI\) 0) Gamet AsB2(SiO.la where Iso. High Grandite often Very pale pink commonest A = R2+ and B = RS + 1.7-1.9 weakly color; inclusions common. birefracting. Indices vary widely with composition. Crystals often euhedraL Uvarovite green, very rare. Staurolite H2FeAI.Si2O'2 Orth. (010) (+) 2V = 87 13=1.750 Low biref. Pleochroic colorless to golden (approximately) (.012) yellow; one good cleavage; twins cruciform or oblique; metamorphic. Olivine Series Mg2SiO. Orth. (+) 2V=85 13=1.651 High biref. Colorless (Fo) to yellow or pale to to (.035) brown (Fa); high relief. Fe2SiO. Orth. (-) 2V=47 13=1.865 High biref. Shagreen (mottled) surface; (.051) often cracked and altered to %II - serpentine. Poor (010) and (100) cleavages. Extinction par- ~ ~ alleL" l~4~ Tourmaline Na(Mg,Fe,Mn,Li,Alk Hex. (-) 111=1.636 Mod. biref. -

GEM NEWS INTERNATIONAL GEMS & GEMOLOGY SUMMER 2015 Sodic Clinopyroxene
Contributing Editors Emmanuel Fritsch, CNRS, Team 6502, Institut des Matériaux Jean Rouxel (IMN), University of Nantes, France (fritsch@cnrs- imn.fr) Kenneth Scarratt, GIA, Bangkok ([email protected]) DIAMOND Diamond-bearing eclogite xenoliths from the Ardo So Ver dykes. Specimens of gem diamond crystals in rock matrix are almost never found due to the rigorous processing that occurs during diamond mining. Occasionally, a diamond is found in a sample of the kimberlite host rock that brought it to the surface, but finding a diamond in the rock in which it likely crystallized in the earth’s mantle is ex- tremely rare. When pieces of the mantle break off and are transported to the surface in a kimberlite magma, they are called xenoliths (meaning “foreign rock,” because the man- tle rocks are not formed from the kimberlite magma itself). Recently, five small rock samples containing diamond crystals (figure 1) were sent to GIA Research for examina- tion. The samples were submitted by Charles Carmona Figure 1. These five diamond-bearing eclogite xeno- (Guild Laboratories, Los Angeles) on behalf of owners Jahn liths from the Ardo So Ver dykes in South Africa Hohne (Ekapa Mining, Kimberley, South Africa) and Vince ranged in weight from 2.24 to 12.40 grams, with the Gerardis (an occasional trader and collector of unique dia- largest measuring 2.5 centimeters in length. Photo by monds and producer of the television series “Game of Kevin Schumacher. Thrones”). The specimens were sourced over 25 years ago from a kimberlite fissure mine located 40 miles (65 km) northwest of Kimberley, South Africa. -

Marialite 3Naalsi3o8 ²Nacl
Marialite 3NaAlSi3O8 ² NaCl c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Tetragonal. Point Group: 4=m: Crystals prismatic, typically with °at pyramidal terminations, striated [001], to 1.5 m; granular, massive. k Physical Properties: Cleavage: 100 , 110 , distinct. Fracture: Uneven to conchoidal. Tenacity: Brittle. Hardness = 5.5{6f D(gmefas.)g= 2.50{2.62 D(calc.) = [2.54] Commonly °uoresces orange to bright yellow or red under LW or SW UV or both. Optical Properties: Transparent to opaque. Color: Colorless, white, grey; pink, violet, blue, yellow, brown, orange-brown; colorless in thin section. Streak: White. Luster: Vitreous, pearly, resinous. Optical Class: Uniaxial ({). ! = 1.539{1.550 ² = 1.532{1.541 Cell Data: Space Group: I4=m: a = 12.060(3) c = 7.572(3) Z = 2 X-ray Powder Pattern: Kenya (?). 3.45 (10), 3.05 (9), 3.80 (8), 2.68 (7), 1.907 (7), 1.363 (6), 2.29 (5) Chemistry: (1) (1) SiO2 60.64 Na2O 12.29 Al2O3 19.56 K2O 0.90 FeO 0.06 Cl 3.9 MgO 0.00 SO3 0.18 CaO 2.66 O = Cl 0.88 ¡ 2 Total 99.31 (1) Kenya (?); by electron microprobe, corresponding to 3[(Na0:80Ca0:13K0:05)§=0:98 Al1:07Si2:83O8] ² (NaCl)0:92: Polymorphism & Series: Forms a series with meionite; intermediate members are P 42=n: Mineral Group: Scapolite group. Occurrence: Typically in regionally metamorphosed rocks, especially marbles, calcareous gneisses, granulites, and greenschists. Also in skarns, some pegmatites, pneumatolytically or hydrothermally altered ma¯c igneous rocks, and ejected volcanic blocks. -

Behaviour in Apatites, Scapolite, and Sodalite
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of Saskatchewan's Research Archive HALOGEN-ELEMENT (F, Cl, AND Br) BEHAVIOUR IN APATITES, SCAPOLITE, AND SODALITE: AN EXPERIMENTAL INVESTIGATION WITH FIELD APPLICATIONS A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Department of Geological Sciences University of Saskatchewan Saskatoon By Ping Dong © Copyright Ping Dong, August, 2005. All Rights Reserved Permission to use In presenting this thesis in partial fulfillment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Geological Sciences University of Saskatchewan 114 Science Place Saskatoon, Saskatchewan Canada S7N 5E2 i Abstract Halogens (F, Cl, Br, and I) are common constituents in igneous, metamorphic, and sedimentary rocks, and are particularly important in the transport of many ore-forming metals. -

The Crystal Chemistry of the Scapolite-Group Minerals. I
1527 The Canadian Mineralogist Vol. 46, pp. 1527-1554 (2008) DOI : 10.3749/canmin.46.6.1527 THE CRYSTAL CHEMISTRY OF THE SCAPOLITE-GROUP MINERALS. I. CRYSTAL STRUCTURE AND LONG-RANGE ORDER EL E NA SOKOLOVA§ AND FRAN K C. HAWTHORNE Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada AB st RA ct Scapolite-group minerals are a group of rock-forming framework aluminosilicates common in a wide variety of igneous and 2+ metamorphic rocks. They have the general formula M4 [T12 O24] A, where M represents Na, K, Ca, Sr, Ba and Fe , T stands for 3+ Si, Al, (Fe ), and A represents Cl, (CO3), (SO4). They constitute a non-binary solid-solution series involving the end members marialite (Na4 [Al3 Si9 O24] Cl, Ma) and meionite (Ca4 [Al6 Si6 O24] CO3, Me). The crystal structures of eighteen scapolite-group 2+ 2+ + minerals from Me4.9 to Me92.8 [Me = 100 SM / S(M + M )] have been refined with MoKa X-radiation. The crystals, a 3 12.045–12.208, c 7.564–7.586 Å, V 1099.7–1130.1 Å , I4/m or P42/n, Z = 2, were analyzed with an electron microprobe subse- quently to collection of the X-ray intensity data. There are subtle differences between the behavior of the distinct T tetrahedra in the I4/m and P42/n structures. For Si and Al, determinative curves for the assignment of site populations from <T–O> distances were developed for each T site in each structure type, and T-site populations were assigned from these curves. -
MM63 321.Pdf
Mineralogical Magazine, June 1999, Vol. 63(3}, pp. 321~329 Silvialite, a new sulfate-dominant member of the scapolite group with an AI-Si composition near the 14/m-P42/n phase transition D. K. TEERTSTRA, M. SCHINDLER, B. L. SHERRIFF AND F. C. HAWTHORNE Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba, Canada R3T 2N2 ABSTRACT Silvialite, ideally Ca4A16Si6024S04, is tetragonal, 141m, Z = 2, with a = 12.160(3), c = 7.560(]) A, v= I] 17.9(8) A3, c:a = 0.6217: 1, (J)= 1.583, I: = 1.558 (uniaxial negative), Dm = 2.75 g/cm3, Deale = 2.769 g/cm3 and H (Mohs) = 5.5. It is transparent and slightly yellow, has a good {IOO} cleavage, chonchoidal fracture, white streak and a vitreous lustre. It occurs in upper-mantle garnet-granulite xenoliths hosted by olivine nephelinite, from McBride Province, North Queensland, Australia. The empirical formula, derived from electron-microprobe analysis, is (Na1.o6Ca2.86)(AI4.87Si7.13)024 [(S04)057(C03)0.4Il Crystal-structure refinement shows disordered carbonate and sulfate groups along the fourfold axis. Silvialite is a primary cumulate phase precipitated from alkali basalt at 900-1000°C and 8 -] 2 kbar under high Is02 and 10,. The name silvialite, currently used in literature to describe the sulfate analogue of meionite, was suggested by Brauns (1914). KEYWORDS: scapolite, meionite, si]vialite, new mineral, sulfate, X-ray data, electron-microprobe data, Queensland, Australia. Introduction Systematic Reference Series of the National Mineral Collection at the Geological Survey of MINERALSof the scapolite group have the general Canada, Ottawa, Ontario, under catalogue number formula M4TI202~, with M = Na, Ca, minor K; T NMC 68080. -
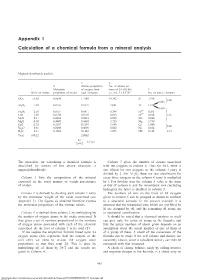
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

41St RMS Program Notes
41st Rochester Mineralogical Symposium 1 Chairman & Emcee - Steve Chamberlain Registrar - Helen Chamberlain Treasurer - Dan Imel Technical Session - Steve Chamberlain Technical Session Moderator - Carl Francis Program Notes, Publicity - Steve Chamberlain Exhibits - Bruce Gaber Program Notes Cover Art - Susan Robinson Dealers - Helen Chamberlain Facilities - Brian McGrath Set-Up/Take-Down Crew - John Diaz, Fred Haynes, Brian McGrath, Bob Morgan, Mike Naven, Lee Tutt, Ken Wolff What’s New In Minerals – Jeff Scovil Auction Solicitors General – Bruce Gaber, Gloria Staebler Auctioneers – Bruce Gaber, Carl Miller Auction Technical Support Wizards - Dan Sperber, Dan Imel Auction Preparation and Support Team - George Robinson, Betty Fetter, Charlene Freundlich, Bill Freundlich, Alicia Imel, Lauren Imel, Dan Sperber, Phyllis Sperber, Laurie Steele-Sperber Registration Desk - Betty Fetter, Susan Robinson, Elizabeth Von Bacho Worthy Keeper of the Micromounters’ Playroom - Quintin Wight Technical and Web Site Support - Dan Imel, Paul Dudley Video - Tom White Security - Bell Security and Investigations Symposium Committee - Helen Chamberlain, Steve Chamberlain, Betty Fetter, Bruce Gaber, Dan Imel, Bob Morgan, Brian McGrath 2 Table of Contents Program . 3 Abstracts of Contributed Papers . 8 Sector Zoning in Diamonds from Zimbabwe by John Rakovan and others . 29 20 Years After: A Brief Update on the Study of Telluride Minerals & Deposits by Bruce Geller . 30 Mineralogical Miracles at Merelani by John Jaszczak . 32 Herodsfoot Mine, Richard Talling and Bournonite by Roy Starkey . 33 The Mount Antero Mineral Locality, Chaffee County, Colorado by Mark I. Jacobson . .37 Minerals of the Cairngorms by Roy Starkey . 40 Our warmest welcome to the 41st Rochester Mineralogical Symposium. We continue this year with our program designed to provide excellent speakers, new information, camaraderie, displays of extraordinary specimens, opportunities to acquire desired objects—all in a familiar and comfortable environment. -

Packing in Ionic Minerals
BULLETIN OF THE GEOLOGICAL SOCIETY OF AMERICA VOL. 54, PP. 1305-1374, 28 FIGS. SEPTEMBER I, 1943 PACKING IN IONIC MINERALS BY H . W. FAIRBAIKN CONTENTS Page Abstract.................................................................................................................................... 1306 Introduction............................................................................................................................ 1307 Acknowledgments.................................................................................................................. 1308 Concept of packing index.................................................................................................... 1308 Development of methods of volume determination.............................................. 1308 The packing index......................................................................................................... 1310 Sources of error.............................................................................................................. 1311 Selection of ion radii..................................................................................................... 1311 Degree of accuracy of packing index........... ............................................................ 1315 Correlation of crystal packing with packing of uniform spheres..................... 1316 Mineralogic correlations of packing index........................................................................ 1320 Correlation of refractive index and density -

Bruce Cairncross
Bruce Cairncross East Africa is famous for its world-class gemstones and mineral specimens, most notably the highly sought-after blue tanzanite, found only in Tanzania in a very small area in the northeast. Many other minerals and gemstones are mined in the region and this is the first full-colour publication to showcase and examine those occurring in the five countries that make up East Africa – Kenya, Tanzania, Rwanda, Uganda and Burundi. The book includes: An introduction to the fascinating geology of the region, including a map depicting the simplified geology and mineral occurrences. Detailed descriptions of minerals, including their gemological properties, history and occurrence. Multiple full-colour photographs of each mineral and gem specimen. Diopside CaMgSi 2O6 Including variety chromian diopside can be faceted into fancy gemstones (Figure 139) and crystals are prized among collectors. Named in 1806 from Greek dis, ‘two’, and opsis, ‘face’. The name refers to the aspects or views in which Chromian diopside is known from Kajiado County in Kenya, where specimens consist of gemmy green the crystal can be oriented. diopside in a blue calcite matrix. The most prized specimens and best gem-quality Crystal system material come from the Merelani Hills in Tanzania Hardness monoclinic where it was first found in 1996. Since then, pockets of crystals have periodically been discovered. The Specific gravity 5.5–6.5 3.22–3.38 chromian diopside is found in layered metasomatic Streak rock in graphite-kyanite gneiss. Crystals can be loose, white, grey Refractive index single or clustered together. Size varies from a few Birefringence 1.664–1.730 millimetres to prismatic crystals of 5–6 cm. -

Appendix a Tables Identification
Appendix A Identification tables These tables summanze information useful in the identification of gemstones. Many of the less common gemstones are not included in the tables. They occur infrequently, so that the need to identify them will arise rarely. Figures quoted in the tables may not agree with those in the body of the text in every case. This is because main text references cover some examples of species with unusual properties, whereas the tables give the constants most likely to be found. Ai SPECIFIC GRA VITY Species Specific gravity Species Specific gravity Gadolinium 7.05 Smithsonite 4.3-4.5 gallium garnet Rutile 4.2-5.6 Cassiterite 6.8-7.0 Spessartine 4.12-4.20 Cerussite 6.45-6.57 Sphalerite 3.9-4.1 Anglesite 6.30-6.39 Celestine 3.97-4.00 Scheelite 5.90-6.10 Almandine 3.95-4.30 Cuprite 5.85-6.15 Zircon (low type) 3.95-4.20 Cubic zirconia 5.6-5.9 Corundum 3.98-4.00 Zincite 5.66-5.68 Willemite 3.89-4.18 Proustite 5.57-5.64 Siderite 3.83-3.96 Strontium titanate 5.13 Demantoid garnet 3.82-3.85 Hematite 4.95-5.16 Azurite 3.77-3.89 Pyrite 4.95-5.10 Malachite 3.60-4.05 Bornite 4.9-5.4 Chrysoberyl 3.71-3.72 Marcasite 4.85-4.92 Pyrope-almandine Zircon (high type) 4.60-4.80 garnet 3.70-3.95 Lithium niobate 4.64 Staurolite 3.65-3.78 YAG 4.57-4.60 Pyrope garnet 3.65-3.70 Baryte 4.3-4.6 Kyanite 3.65-3.68 338 Appendix A Identification tables Species Specific gravity Species Specific gravity Grossular garnet 3.65 Brazilianite 2.98-2.99 Behitoite 3.64-3.68 Boracite 2.95 Spinel (synthetic) 3.61-3.65 Phenakite 2.93-2.97 Taaffeite 3.60-3.61